Convalescent Plasma for Patients Hospitalized With Coronavirus Disease 2019: A Meta-Analysis With Trial Sequential Analysis of Randomized Controlled Trials
- PMID: 34782209
- PMCID: PMC8502250
- DOI: 10.1016/j.tmrv.2021.09.001
Convalescent Plasma for Patients Hospitalized With Coronavirus Disease 2019: A Meta-Analysis With Trial Sequential Analysis of Randomized Controlled Trials
Abstract
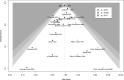
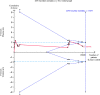
Current evidence from randomized controlled trials (RCTs) and systematic reviews on the utility of convalescent plasma (CP) in patients with coronavirus disease 2019 (COVID-19) suggests a lack of benefit. We conducted an updated meta-analysis of RCTs with trial sequential analysis to investigate whether convalescent plasma is futile in reducing mortality in patients hospitalized with COVID-19. We searched 6 databases from December 1, 2019 to August 1, 2021 for RCTs comparing the use of CP with standard of care or transfusion of non-CP standard plasma in patients with COVID-19. The risk of bias was assessed using the Cochrane Risk-of-Bias 2 Tool. Random effects (DerSimonian and Laird) meta-analyses were conducted. The primary outcome was the aggregate risk for in-hospital mortality between both arms. We conducted a trial sequential analysis (TSA) based on the pooled relative risks (RRs) for in-hospital mortality. Secondary outcomes included the pooled RR for receipt of mechanical ventilation and mean difference in hospital length of stay. We included 18 RCTs (8702 CP, 7906 control). CP was not associated with a significant mortality benefit (RR: 0.95, 95%-CI: 0.86-1.04, P = .27, high certainty). Subgroup analysis did not find any significant differences (pinteraction = 0.30) between patients who received CP within 8 days of symptom onset (RR: 0.97, 95%-CI: 0.79-1.19, P = .80), or after 8 days (RR: 0.79, 95%-CI: 0.57-1.10, P = .16). TSA based on a RR reduction of 10% from a baseline mortality of 20% found that CP was not effective, with the pooled effect within the boundary for futility. CP did not significantly reduce the requirement for mechanical ventilation (RR: 1.00, 95%-CI: 0.91-1.10, P = .99, moderate certainty) or hospital length of stay (+1.32, 95%-CI: -1.86 to +4.52, P = .42, low certainty). CP does not improve relevant clinical outcomes in patients with COVID-19, especially in severe disease. The pooled effect of mortality was within the boundary of futility, suggesting the lack of benefit of CP in patients hospitalized with COVID-19.
Keywords: Convalescent plasma; Coronavirus disease 2019; Meta-analysis; Mortality; Severe acute respiratory syndrome coronavirus 2.
Copyright © 2021. Published by Elsevier Inc.
Conflict of interest statement
All authors declare no competing interests.
Figures


Similar articles
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2021 May 20;5(5):CD013600. doi: 10.1002/14651858.CD013600.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2023 Feb 1;2:CD013600. doi: 10.1002/14651858.CD013600.pub5. PMID: 34013969 Free PMC article. Updated.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2021 May 20;5:CD013600. doi: 10.1002/14651858.CD013600.pub4. PMID: 33044747 Updated.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review.Cochrane Database Syst Rev. 2020 Jul 10;7(7):CD013600. doi: 10.1002/14651858.CD013600.pub2. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Oct 12;10:CD013600. doi: 10.1002/14651858.CD013600.pub3. PMID: 32648959 Free PMC article. Updated.
-
Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a rapid review.Cochrane Database Syst Rev. 2020 May 14;5(5):CD013600. doi: 10.1002/14651858.CD013600. Cochrane Database Syst Rev. 2020. Update in: Cochrane Database Syst Rev. 2020 Jul 10;7:CD013600. doi: 10.1002/14651858.CD013600.pub2. PMID: 32406927 Free PMC article. Updated.
-
CONVALESCENT plasma for COVID-19: A meta-analysis of clinical trials and real-world evidence.Eur J Clin Invest. 2021 Nov;51(11):e13663. doi: 10.1111/eci.13663. Epub 2021 Aug 18. Eur J Clin Invest. 2021. PMID: 34375445 Free PMC article.
Cited by
-
The efficiency of convalescent plasma in COVID-19 patients: A systematic review and meta-analysis of randomized controlled clinical trials.Front Immunol. 2022 Jul 28;13:964398. doi: 10.3389/fimmu.2022.964398. eCollection 2022. Front Immunol. 2022. PMID: 35967398 Free PMC article.
-
Hyperimmune Plasma and Immunoglobulins against COVID-19: A Narrative Review.Life (Basel). 2024 Feb 1;14(2):214. doi: 10.3390/life14020214. Life (Basel). 2024. PMID: 38398723 Free PMC article. Review.
-
ERS International Congress 2021: highlights from the Respiratory Clinical Care and Physiology Assembly.ERJ Open Res. 2022 May 23;8(2):00710-2021. doi: 10.1183/23120541.00710-2021. eCollection 2022 Apr. ERJ Open Res. 2022. PMID: 35615417 Free PMC article. Review.
-
Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: a randomized controlled trial (PROTECT-Patient Trial).Sci Rep. 2022 Feb 15;12(1):2552. doi: 10.1038/s41598-022-06221-8. Sci Rep. 2022. PMID: 35169169 Free PMC article. Clinical Trial.
-
Multi-institutional experience with COVID-19 convalescent plasma in children.Transfusion. 2023 May;63(5):918-924. doi: 10.1111/trf.17318. Epub 2023 Mar 30. Transfusion. 2023. PMID: 36965173 Free PMC article.
References
-
- Ling R.R., Ramanathan K., Tan W.Q., Yeo L.H.Y., Poon W.H., Syn N.L., et al. Interleukin-6 receptor antagonists for severe coronavirus disease 2019: a meta-analysis of reconstructed individual patient data from randomised controlled trials. SSRN. 2021 doi: 10.2139/ssrn.3844782. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

