Mechanical Stress Signaling in Pancreatic Cancer Cells Triggers p38 MAPK- and JNK-Dependent Cytoskeleton Remodeling and Promotes Cell Migration via Rac1/cdc42/Myosin II
- PMID: 34782370
- PMCID: PMC8898300
- DOI: 10.1158/1541-7786.MCR-21-0266
Mechanical Stress Signaling in Pancreatic Cancer Cells Triggers p38 MAPK- and JNK-Dependent Cytoskeleton Remodeling and Promotes Cell Migration via Rac1/cdc42/Myosin II
Abstract
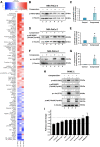
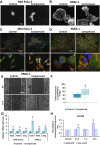
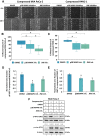
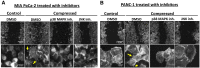
Advanced or metastatic pancreatic cancer is highly resistant to existing therapies, and new treatments are urgently needed to improve patient outcomes. Current studies focus on alternative treatment approaches that target the abnormal microenvironment of pancreatic tumors and the resulting elevated mechanical stress in the tumor interior. Nevertheless, the underlying mechanisms by which mechanical stress regulates pancreatic cancer metastatic potential remain elusive. Herein, we used a proteomic assay to profile mechanical stress-induced signaling cascades that drive the motility of pancreatic cancer cells. Proteomic analysis, together with selective protein inhibition and siRNA treatments, revealed that mechanical stress enhances cell migration through activation of the p38 MAPK/HSP27 and JNK/c-Jun signaling axes, and activation of the actin cytoskeleton remodelers: Rac1, cdc42, and myosin II. In addition, mechanical stress upregulated transcription factors associated with epithelial-to-mesenchymal transition and stimulated the formation of stress fibers and filopodia. p38 MAPK and JNK inhibition resulted in lower cell proliferation and more effectively blocked cell migration under mechanical stress compared with control conditions. The enhanced tumor cell motility under mechanical stress was potently reduced by cdc42 and Rac1 silencing with no effects on proliferation. Our results highlight the importance of targeting aberrant signaling in cancer cells that have adapted to mechanical stress in the tumor microenvironment, as a novel approach to effectively limit pancreatic cancer cell migration.
Implications: Our findings highlight that mechanical stress activated the p38 MAPK and JNK signaling axis and stimulated pancreatic cancer cell migration via upregulation of the actin cytoskeleton remodelers cdc42 and Rac1.
©2021 The Authors; Published by the American Association for Cancer Research.
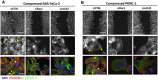
Figures


References
-
- Aier I, Semwal R, Sharma A, Varadwaj PK. A systematic assessment of statistics, risk factors, and underlying features involved in pancreatic cancer. Cancer Epidemiol 2019;58:104–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

