Dopaminergic Therapy for Motor Symptoms in Early Parkinson Disease Practice Guideline Summary: A Report of the AAN Guideline Subcommittee
- PMID: 34782410
- PMCID: PMC8672433
- DOI: 10.1212/WNL.0000000000012868
Dopaminergic Therapy for Motor Symptoms in Early Parkinson Disease Practice Guideline Summary: A Report of the AAN Guideline Subcommittee
Abstract
Background and objectives: To review the current evidence on the options available for initiating dopaminergic treatment of motor symptoms in early-stage Parkinson disease and provide recommendations to clinicians.
Methods: A multidisciplinary panel developed practice recommendations, integrating findings from a systematic review and following an Institute of Medicine-compliant process to ensure transparency and patient engagement. Recommendations were supported by structured rationales, integrating evidence from the systematic review, related evidence, principles of care, and inferences from evidence.
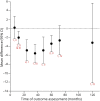
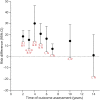
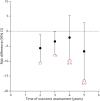
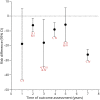
Results: Initial treatment with levodopa provides superior motor benefit compared to treatment with dopamine agonists, whereas levodopa is more likely than dopamine agonists to cause dyskinesia. The comparison of different formulations of dopamine agonists yielded little evidence that any one formulation or method of administration is superior. Long-acting forms of levodopa and levodopa with entacapone do not appear to differ in efficacy from immediate-release levodopa for motor symptoms in early disease. There is a higher risk of impulse control disorders associated with the use of dopamine agonists than levodopa. Recommendations on initial therapy for motor symptoms are provided to assist the clinician and patient in choosing between treatment options and to guide counseling, prescribing, and monitoring of efficacy and safety.
© 2021 American Academy of Neurology.
Figures
References
-
- Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson's disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2002;58(1):11-17. - PubMed
-
- Gronseth GS, Cox J, Gloss D, et al. . Clinical Practice Guideline Process Manual, 2nd Edition. American Academy of Neurology; 2017. Accessed June 9, 2021. aan.com/siteassets/home-page/policy-and-guidelines/guidelines/about-guid...
-
- Gronseth GS, Woodroffe LM, Getchius TSD. Clinical Practice Guideline Process Manual, 2011 Edition. American Academy of Neurology; 2011. Accessed June 9, 2021. aan.com/siteassets/home-page/policy-and-guidelines/guidelines/about-guid...
-
- Caraceni T, Musicco M. Levodopa or dopamine agonists, or deprenyl as initial treatment for Parkinson's disease. A randomized multicenter study. Parkinsonism Relat Disord. 2001;7(2):107-114. - PubMed
-
- PD Med Collaborative Group, Gray R, Ives N, Rick C, et al. . Long-term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): a large, open-label, pragmatic randomised trial. Lancet. 2014;384(9949):1196-1205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
