Cerebral Microbleeds and Treatment Effect of Intravenous Thrombolysis in Acute Stroke: An Analysis of the WAKE-UP Randomized Clinical Trial
- PMID: 34782419
- PMCID: PMC8792812
- DOI: 10.1212/WNL.0000000000013055
Cerebral Microbleeds and Treatment Effect of Intravenous Thrombolysis in Acute Stroke: An Analysis of the WAKE-UP Randomized Clinical Trial
Abstract
Background and objectives: Cerebral microbleeds (CMBs) are common in patients with acute ischemic stroke and are associated with increased risk of intracerebral hemorrhage (ICH) after intravenous thrombolysis. Whether CMBs modify the treatment effect of thrombolysis is unknown.
Methods: We performed a prespecified analysis of the prospective randomized controlled multicenter Efficacy and Safety of MRI-Based Thrombolysis in Wake-Up Stroke (WAKE-UP) trial including patients with acute ischemic stroke with unknown time of symptom onset and diffusion-weighted imaging-fluid-attenuated inversion recovery mismatch on MRI receiving alteplase or placebo. Patients were screened and enrolled between September 2012 and June 2017 (with final follow-up in September 2017). Patients were randomized to treatment with IV thrombolysis with alteplase at 0.9 mg/kg body weight or placebo. CMB status (presence, number, and distribution) was assessed after study completion by 3 raters blinded to clinical information following a standardized protocol. Outcome measures were excellent functional outcome at 90 days, defined by modified Rankin Scale (mRS) score ≤1, and symptomatic ICH according to National Institutes of Neurological Disease and Stroke trial criteria 22 to 36 hours after treatment.
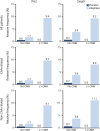
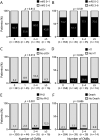
Results: Of 503 patients enrolled in the WAKE-UP trial, 459 (91.3%; 288 [63%] men) were available for analysis. Ninety-eight (21.4%) had at least 1 CMB on baseline imaging; 45 (9.8%) had exactly 1 CMB; 37 (8.1%) had 2 to 4 CMBs; and 16 (3.5%) had ≥5 CMBs. Presence of CMBs was associated with a nonsignificant increased risk of symptomatic ICH (11.2% vs 4.2%; adjusted odds ratio [OR] 2.32, 95% confidence interval [CI] 0.99-5.43, p = 0.052) but had no effect on functional outcome at 90 days (mRS score ≤1: 45.8% vs 50.7%; adjusted OR 0.99, 95% CI 0.59-1.64, p = 0.955). Patients receiving alteplase had better functional outcome (mRS score ≤1: 54.6% vs 44.6%, adjusted OR 1.61, 95% CI 1.07-2.43, p = 0.022) without evidence of heterogeneity in relation to CMB presence (p of the interactive term = 0.546). Results were similar for subpopulations with strictly lobar (presumed cerebral amyloid angiopathy related) or not strictly lobar CMB distribution.
Discussion: In the randomized-controlled WAKE-UP trial, we saw no evidence of reduced treatment effect of alteplase in patients with acute ischemic stroke with ≥1 CMBs. Additional studies are needed to determine the treatment effect of alteplase and its benefit-harm ratio in patients with a larger number of CMBs.
Trial registration information: ClinicalTrials.gov identifier NCT01525290; ClinicalTrialsRegister.EU identifier 2011-005906-32.
Classification of evidence: This study provides Class II evidence that for patients with acute ischemic stroke with unknown time of onset and diffusion-weighted imaging-fluid-attenuated inversion recovery mismatch who received IV alteplase, CMBs are not significantly associated with functional outcome at 90 days.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



Comment in
-
Author Response: Cerebral Microbleeds and Treatment Effect of Intravenous Thrombolysis in Acute Stroke: An Analysis of the WAKE-UP Randomized Clinical Trial.Neurology. 2022 May 10;98(19):817. doi: 10.1212/WNL.0000000000200612. Neurology. 2022. PMID: 35534239 No abstract available.
-
Reader Response: Cerebral Microbleeds and Treatment Effect of Intravenous Thrombolysis in Acute Stroke: An Analysis of the WAKE-UP Randomized Clinical Trial.Neurology. 2022 May 10;98(19):816-817. doi: 10.1212/WNL.0000000000200611. Neurology. 2022. PMID: 35534240 No abstract available.
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. - PubMed
-
- Charidimou A, Turc G, Oppenheim C, et al. . Microbleeds, cerebral hemorrhage, and functional outcome after stroke thrombolysis. Stroke. 2017;48(8):2084-2090. - PubMed
-
- Tsivgoulis G, Zand R, Katsanos AH, et al. . Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with acute ischemic stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol. 2016;73(6):675-683. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
