Staphylococcal ClpXP protease targets the cellular antioxidant system to eliminate fitness-compromised cells in stationary phase
- PMID: 34782466
- PMCID: PMC8617462
- DOI: 10.1073/pnas.2109671118
Staphylococcal ClpXP protease targets the cellular antioxidant system to eliminate fitness-compromised cells in stationary phase
Abstract
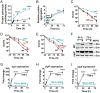
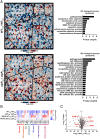
The transition from growth to stationary phase is a natural response of bacteria to starvation and stress. When stress is alleviated and more favorable growth conditions return, bacteria resume proliferation without a significant loss in fitness. Although specific adaptations that enhance the persistence and survival of bacteria in stationary phase have been identified, mechanisms that help maintain the competitive fitness potential of nondividing bacterial populations have remained obscure. Here, we demonstrate that staphylococci that enter stationary phase following growth in media supplemented with excess glucose, undergo regulated cell death to maintain the competitive fitness potential of the population. Upon a decrease in extracellular pH, the acetate generated as a byproduct of glucose metabolism induces cytoplasmic acidification and extensive protein damage in nondividing cells. Although cell death ensues, it does not occur as a passive consequence of protein damage. Instead, we demonstrate that the expression and activity of the ClpXP protease is induced, resulting in the degeneration of cellular antioxidant capacity and, ultimately, cell death. Under these conditions, inactivation of either clpX or clpP resulted in the extended survival of unfit cells in stationary phase, but at the cost of maintaining population fitness. Finally, we show that cell death from antibiotics that interfere with bacterial protein synthesis can also be partly ascribed to the corresponding increase in clpP expression and activity. The functional conservation of ClpP in eukaryotes and bacteria suggests that ClpP-dependent cell death and fitness maintenance may be a widespread phenomenon in these domains of life.
Keywords: Staphylococcus aureus; clpP; clpX; sodA; stationary phase fitness.
Conflict of interest statement
The authors declare no competing interest.
Figures




Comment in
-
Bacteria clip out damaged cells from populations.Proc Natl Acad Sci U S A. 2021 Dec 7;118(49):e2118892118. doi: 10.1073/pnas.2118892118. Proc Natl Acad Sci U S A. 2021. PMID: 34848543 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

