Toxin secretion and trafficking by Mycobacterium tuberculosis
- PMID: 34782620
- PMCID: PMC8593097
- DOI: 10.1038/s41467-021-26925-1
Toxin secretion and trafficking by Mycobacterium tuberculosis
Abstract
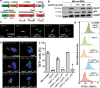
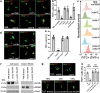
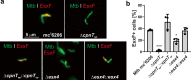
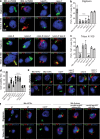
The tuberculosis necrotizing toxin (TNT) is the major cytotoxicity factor of Mycobacterium tuberculosis (Mtb) in macrophages. TNT is the C-terminal domain of the outer membrane protein CpnT and gains access to the cytosol to kill macrophages infected with Mtb. However, molecular mechanisms of TNT secretion and trafficking are largely unknown. A comprehensive analysis of the five type VII secretion systems of Mtb revealed that the ESX-4 system is required for export of CpnT and surface accessibility of TNT. Furthermore, the ESX-2 and ESX-4 systems are required for permeabilization of the phagosomal membrane in addition to the ESX-1 system. Thus, these three ESX systems need to act in concert to enable trafficking of TNT into the cytosol of Mtb-infected macrophages. These discoveries establish new molecular roles for the two previously uncharacterized type VII secretion systems ESX-2 and ESX-4 and reveal an intricate link between toxin secretion and phagosomal permeabilization by Mtb.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Schiavo G, van der Goot FG. The bacterial toxin toolkit. Nat. Rev. Mol. Cell Biol. 2001;2:530–537. - PubMed
-
- Bischofberger M, van der Goot FG. Exotoxin secretion: getting out to find the way in. Cell Host Microbe. 2008;3:7–8. - PubMed
-
- Rapisarda C, Fronzes R. Secretion systems used by bacteria to subvert host functions. Curr. Issues Mol. Biol. 2018;25:1–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

