Supplying the trip to antibody production-nutrients, signaling, and the programming of cellular metabolism in the mature B lineage
- PMID: 34782762
- PMCID: PMC8591438
- DOI: 10.1038/s41423-021-00782-w
Supplying the trip to antibody production-nutrients, signaling, and the programming of cellular metabolism in the mature B lineage
Abstract
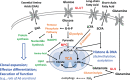
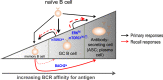
The COVID pandemic has refreshed and expanded recognition of the vital role that sustained antibody (Ab) secretion plays in our immune defenses against microbes and of the importance of vaccines that elicit Ab protection against infection. With this backdrop, it is especially timely to review aspects of the molecular programming that govern how the cells that secrete Abs arise, persist, and meet the challenge of secreting vast amounts of these glycoproteins. Whereas plasmablasts and plasma cells (PCs) are the primary sources of secreted Abs, the process leading to the existence of these cell types starts with naive B lymphocytes that proliferate and differentiate toward several potential fates. At each step, cells reside in specific microenvironments in which they not only receive signals from cytokines and other cell surface receptors but also draw on the interstitium for nutrients. Nutrients in turn influence flux through intermediary metabolism and sensor enzymes that regulate gene transcription, translation, and metabolism. This review will focus on nutrient supply and how sensor mechanisms influence distinct cellular stages that lead to PCs and their adaptations as factories dedicated to Ab secretion. Salient findings of this group and others, sometimes exhibiting differences, will be summarized with regard to the journey to a distinctive metabolic program in PCs.
Keywords: B lymphocyte; Fatty acid; Glucose; Glutamine; Intermediary metabolism; Plasma cell; Signal transduction.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Jellusova J. Cross-talk between signal transduction and metabolism in B cells. Immunol Lett. 2018;201:1–13. - PubMed
-
- Franchina DG, Grusdat M, Brenner D. B-cell metabolic remodeling and cancer. Trends Cancer. 2018;4:138–50. - PubMed
-
- Jellusova J. Metabolic control of B cell immune responses. Curr Opin Immunol. 2020;63:21–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

