Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19
- PMID: 34782790
- PMCID: PMC8799469
- DOI: 10.1038/s41591-021-01576-3
Dexamethasone modulates immature neutrophils and interferon programming in severe COVID-19
Abstract
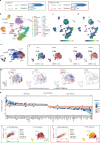
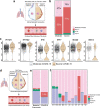
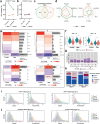
Although critical for host defense, innate immune cells are also pathologic drivers of acute respiratory distress syndrome (ARDS). Innate immune dynamics during Coronavirus Disease 2019 (COVID-19) ARDS, compared to ARDS from other respiratory pathogens, is unclear. Moreover, mechanisms underlying the beneficial effects of dexamethasone during severe COVID-19 remain elusive. Using single-cell RNA sequencing and plasma proteomics, we discovered that, compared to bacterial ARDS, COVID-19 was associated with expansion of distinct neutrophil states characterized by interferon (IFN) and prostaglandin signaling. Dexamethasone during severe COVID-19 affected circulating neutrophils, altered IFNactive neutrophils, downregulated interferon-stimulated genes and activated IL-1R2+ neutrophils. Dexamethasone also expanded immunosuppressive immature neutrophils and remodeled cellular interactions by changing neutrophils from information receivers into information providers. Male patients had higher proportions of IFNactive neutrophils and preferential steroid-induced immature neutrophil expansion, potentially affecting outcomes. Our single-cell atlas (see 'Data availability' section) defines COVID-19-enriched neutrophil states and molecular mechanisms of dexamethasone action to develop targeted immunotherapies for severe COVID-19.
© 2021. The Author(s).
Conflict of interest statement
S.S. declares stock ownership in 10x Genomics. All other authors declare no conflicts of interest.
Figures










Comment in
-
Dexamethasone restrains neutrophils in severe COVID-19.Nat Rev Immunol. 2022 Jan;22(1):5. doi: 10.1038/s41577-021-00669-x. Nat Rev Immunol. 2022. PMID: 34862486 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

