Inclusion of hepatitis C virus testing in National Health Screening to accelerate HCV elimination in South Korea
- PMID: 34782871
- PMCID: PMC8562097
- DOI: 10.35772/ghm.2021.01057
Inclusion of hepatitis C virus testing in National Health Screening to accelerate HCV elimination in South Korea
Abstract
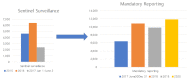
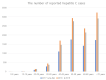
In 2015, the World Health Organization (WHO) has set the goal of eliminating hepatitis C by reducing incidence of chronic viral hepatitis and related mortality by 2030 with the interim target of achieving 30% prevalence reduction by 2020. While The global prevalence of hepatitis C is known to be around 1.6%, the prevalence of hepatitis C in South Korea is 0.5-0.6% based on hepatitis C virus (HCV) antibody-positive rate. Although HCV antibody test has been included in the Annual National Health and Nutrition Survey in South Korea since 2012, a national initiative to eliminate hepatitis C was initiated by small clinic-related hepatitis C outbreaks in 2015-2016. These outbreaks caused by inappropriate use of syringes in 2015-2016 prompted the revision of hepatitis C reporting and control strategies in Korea following long-term discussion on including the HCV antibody test in the National Health Screening at a certain age. Since June 3, 2017, all hepatitis C cases should be reported to the Korea Disease Control Agency (KDCA). A pilot study for early detection of hepatitis C was conducted for the 56 years old population from September 1 to October 31 in 2020 by temporarily including HCV Ab in the National Health Screening followed by HCV RNA testing for HCV antibody positive cases. The final decision to include HCV antibody test in National Health Screening will be made based on results of the pilot study in 2020. To eliminate hepatitis B & C by 2030 in South Korea, the KDCA established a comprehensive viral hepatitis control and management system in 2020 with the interim goal of achieving an antibody positive rate of 0.3% and treatment rate of 90% by 2025.
Keywords: National Health and Nutrition Survey; hepatitis C elimination; hepatitis C virus RNA; hepatitis C virus antibody; treatment.
2021, National Center for Global Health and Medicine.
Conflict of interest statement
The author has no conflicts of interest to disclose.
Figures


References
-
- World Health Organization. Global Health Sector Strategy on Viral Hepatitis 2016-2021. http://apps.who.int/iris/bitstream/handle/10665/246177/WHO-HIV-2016.06-e... (accessed May 16, 2021).
-
- Pedrana A, Howell J, Scott N, et al. . Global hepatitis C elimination: an investment framework. Lancet Gastroenterol Hepatol. 2020; 5:927-939. - PubMed
-
- Waked I, Esmat G, Elsharkawy A, et al. . Screening and treatment program to eliminate hepatitis C in Egypt. N Engl J Med. 2020; 382:1166-1174. - PubMed
-
- Radley A, de Bruin M, Inglis SK, Donnan PT, Hapca A, Barclay ST, Fraser A, Dillon JF. Clinical effectiveness of pharmacist-led versus conventionally delivered antiviral treatment for hepatitis C virus in patients receiving opioid substitution therapy: a pragmatic, cluster-randomised trial. Lancet Gasteroenterol Hepatol. 2020; 5:809-818. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

