d-Galactose induced early aging in human erythrocytes: Role of band 3 protein
- PMID: 34783011
- PMCID: PMC9299479
- DOI: 10.1002/jcp.30632
d-Galactose induced early aging in human erythrocytes: Role of band 3 protein
Abstract
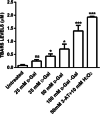
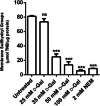
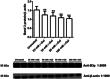
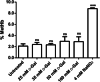
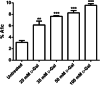
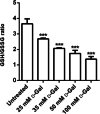
Aging, a time-dependent multifaceted process, affects both cell structure and function and involves oxidative stress as well as glycation. The present investigation focuses on the role of the band 3 protein (B3p), an anion exchanger essential to red cells homeostasis, in a d-galactose ( d-Gal)-induced aging model. Anion exchange capability, measured by the rate constant of SO₄²- uptake through B3p, levels of lipid peroxidation, oxidation of membrane sulfhydryl groups, B3p expression, methemoglobin, glycated hemoglobin (Hb), and the reduced glutathione/oxidized glutathione ratio were determined after exposure of human erythrocytes to 25, 35, 50, and 100 mmol/L d-Gal for 24 h. Our results show that: (i) in vitro application of d-Gal is useful to model early aging in human erythrocytes; (ii) assessment of B3p ion transport function is a sensitive tool to monitor aging development; (iii) d-Gal leads to Hb glycation and produces substantial changes on the endogenous antioxidant system; (iv) the impact of aging on B3p function proceeds through steps, first involving Hb glycation and then oxidative events at the membrane level. These findings offer a useful tool to understand the mechanisms of aging in human erythrocytes and propose B3p as a possible target for new therapeutic strategies to counteract age-related disturbances.
Keywords: aging; anion exchange; band 3 protein; d-galactose; erythrocytes; glycation; oxidative stress.
© 2021 The Authors. Journal of Cellular Physiology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that there are no conflict of interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

