Heme oxygenase-1/carbon monoxide signaling participates in the accumulation of triterpenoids of Ganoderma lucidum
- PMID: 34783224
- PMCID: PMC8593529
- DOI: 10.1631/jzus.B2000818
Heme oxygenase-1/carbon monoxide signaling participates in the accumulation of triterpenoids of Ganoderma lucidum
Abstract
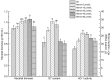
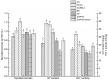
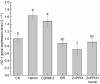
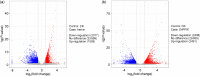
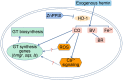
Ganoderic triterpenoids (GTs) are the primary bioactive constituents of the Basidiomycotina fungus, Ganoderma lucidum. These compounds exhibit antitumor, anti-hyperlipidemic, and immune-modulatory pharmacological activities. This study focused on GT accumulation in mycelia of G. lucidum mediated by the heme oxygenase-1 (HO-1)/carbon monoxide (CO) signaling. Compared with the control, hemin (10 μmol/L) induced an increase of 60.1% in GT content and 57.1% in HO-1 activity. Moreover, carbon monoxide-releasing molecule-2 (CORM-2), CO donor, increased GT content by 56.0% and HO-1 activity by 18.1%. Zn protoporphyrin IX (ZnPPIX), a specific HO-1 inhibitor, significantly reduced GT content by 26.0% and HO-1 activity by 15.8%, while hemin supplementation reversed these effects. Transcriptome sequencing showed that HO-1/CO could function directly as a regulator involved in promoting GT accumulation by regulating gene expression in the mevalonate pathway, and modulating the reactive oxygen species (ROS) and Ca2+ pathways. The results of this study may help enhance large-scale GT production and support further exploration of GT metabolic networks and relevant signaling cross-talk.
Keywords: Ganoderma lucidum; Heme oxygenase-1 (HO-1)/carbon monoxide (CO) signaling; Transcriptome sequencing; Triterpenoid.
Figures
Similar articles
-
Heme-oxygenase-1 induction and carbon monoxide-releasing molecule inhibit lipopolysaccharide (LPS)-induced high-mobility group box 1 release in vitro and improve survival of mice in LPS- and cecal ligation and puncture-induced sepsis model in vivo.Mol Pharmacol. 2009 Jul;76(1):173-82. doi: 10.1124/mol.109.055137. Epub 2009 Apr 14. Mol Pharmacol. 2009. PMID: 19366789
-
Anti-inflammatory and heme oxygenase-1 inducing activities of lanostane triterpenes isolated from mushroom Ganoderma lucidum in RAW264.7 cells.Toxicol Appl Pharmacol. 2014 Nov 1;280(3):434-42. doi: 10.1016/j.taap.2014.09.007. Epub 2014 Sep 16. Toxicol Appl Pharmacol. 2014. PMID: 25239868
-
Carbon monoxide contributes to the constipating effects of granisetron in rat colon.World J Gastroenterol. 2016 Nov 14;22(42):9333-9345. doi: 10.3748/wjg.v22.i42.9333. World J Gastroenterol. 2016. PMID: 27895421 Free PMC article.
-
Heme oxygenase-1: a metabolic nike.Antioxid Redox Signal. 2014 Apr 10;20(11):1709-22. doi: 10.1089/ars.2013.5667. Epub 2014 Feb 27. Antioxid Redox Signal. 2014. PMID: 24180257 Free PMC article. Review.
-
Therapeutic potential and nutritional significance of Ganoderma lucidum - a comprehensive review from 2010 to 2022.Food Funct. 2023 Feb 21;14(4):1812-1838. doi: 10.1039/d2fo01683d. Food Funct. 2023. PMID: 36734035
Cited by
-
Heme Oxygenase/Carbon Monoxide Participates in the Regulation of Ganoderma lucidum Heat-Stress Response, Ganoderic Acid Biosynthesis, and Cell-Wall Integrity.Int J Mol Sci. 2022 Oct 29;23(21):13147. doi: 10.3390/ijms232113147. Int J Mol Sci. 2022. PMID: 36361934 Free PMC article.
-
Understanding the characteristic changes of retrogradation behavior and edible quality of brown rice modified with inhibiting retrogradation enzymes of Ganoderma lucidum.Curr Res Food Sci. 2024 Nov 15;9:100927. doi: 10.1016/j.crfs.2024.100927. eCollection 2024. Curr Res Food Sci. 2024. PMID: 39640018 Free PMC article.
References
-
- Cao ZY, Huang BK, Wang QY, et al. , 2007. Involvement of carbon monoxide produced by heme oxygenase in ABA-induced stomatal closure in Vicia faba and its proposed signal transduction pathway. Chin Sci Bull, 52(17): 2365-2373. 10.1007/s11434-007-0358-y - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

