Maturation of persistent and hyperpolarization-activated inward currents shapes the differential activation of motoneuron subtypes during postnatal development
- PMID: 34783651
- PMCID: PMC8641952
- DOI: 10.7554/eLife.71385
Maturation of persistent and hyperpolarization-activated inward currents shapes the differential activation of motoneuron subtypes during postnatal development
Abstract
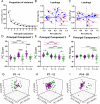
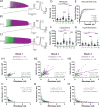
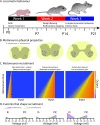
The size principle underlies the orderly recruitment of motor units; however, motoneuron size is a poor predictor of recruitment amongst functionally defined motoneuron subtypes. Whilst intrinsic properties are key regulators of motoneuron recruitment, the underlying currents involved are not well defined. Whole-cell patch-clamp electrophysiology was deployed to study intrinsic properties, and the underlying currents, that contribute to the differential activation of delayed and immediate firing motoneuron subtypes. Motoneurons were studied during the first three postnatal weeks in mice to identify key properties that contribute to rheobase and may be important to establish orderly recruitment. We find that delayed and immediate firing motoneurons are functionally homogeneous during the first postnatal week and are activated based on size, irrespective of subtype. The rheobase of motoneuron subtypes becomes staggered during the second postnatal week, which coincides with the differential maturation of passive and active properties, particularly persistent inward currents. Rheobase of delayed firing motoneurons increases further in the third postnatal week due to the development of a prominent resting hyperpolarization-activated inward current. Our results suggest that motoneuron recruitment is multifactorial, with recruitment order established during postnatal development through the differential maturation of passive properties and sequential integration of persistent and hyperpolarization-activated inward currents.
Keywords: intrinsic properties; motoneuron; mouse; neuroscience; postnatal development; recruitment; size principle; spinal cord.
© 2021, Sharples and Miles.
Conflict of interest statement
SS, GM No competing interests declared
Figures






Similar articles
-
Postnatal development of persistent inward currents in rat XII motoneurons and their modulation by serotonin, muscarine and noradrenaline.J Physiol. 2019 Jun;597(12):3183-3201. doi: 10.1113/JP277572. Epub 2019 May 22. J Physiol. 2019. PMID: 31038198
-
Can local vibration alter the contribution of persistent inward currents to human motoneuron firing?J Physiol. 2023 Apr;601(8):1467-1482. doi: 10.1113/JP284210. Epub 2023 Mar 22. J Physiol. 2023. PMID: 36852473
-
Potassium currents dynamically set the recruitment and firing properties of F-type motoneurons in neonatal mice.J Neurophysiol. 2015 Sep;114(3):1963-73. doi: 10.1152/jn.00193.2015. Epub 2015 Aug 12. J Neurophysiol. 2015. PMID: 26269551 Free PMC article.
-
Integration of synaptic and intrinsic dendritic currents in cat spinal motoneurons.Brain Res Brain Res Rev. 2002 Oct;40(1-3):1-8. doi: 10.1016/s0165-0173(02)00183-2. Brain Res Brain Res Rev. 2002. PMID: 12589901 Review.
-
Postnatal development of hypoglossal motoneuron intrinsic properties.Adv Exp Med Biol. 1995;381:63-71. doi: 10.1007/978-1-4615-1895-2_7. Adv Exp Med Biol. 1995. PMID: 8867824 Review.
Cited by
-
Mechanisms Underlying the Recruitment of Inhibitory Interneurons in Fictive Swimming in Developing Xenopus laevis Tadpoles.J Neurosci. 2023 Feb 22;43(8):1387-1404. doi: 10.1523/JNEUROSCI.0520-22.2022. Epub 2023 Jan 24. J Neurosci. 2023. PMID: 36693757 Free PMC article.
-
Intrinsic motoneuron properties in typical human development.J Physiol. 2024 May;602(9):2061-2087. doi: 10.1113/JP285756. Epub 2024 Mar 30. J Physiol. 2024. PMID: 38554126 Free PMC article.
-
Age-related increase in the excitability of mouse layer V pyramidal neurons in the primary motor cortex is accompanied by an increased persistent inward current.Geroscience. 2025 Apr;47(2):2199-2222. doi: 10.1007/s11357-024-01405-8. Epub 2024 Oct 30. Geroscience. 2025. PMID: 39472350 Free PMC article.
-
Spinal microcircuits go through multiphasic homeostatic compensations in a mouse model of motoneuron degeneration.Cell Rep. 2024 Dec 24;43(12):115046. doi: 10.1016/j.celrep.2024.115046. Epub 2024 Dec 9. Cell Rep. 2024. PMID: 39656589 Free PMC article.
-
On the origin of F-wave: involvement of central synaptic mechanisms.Brain. 2024 Feb 1;147(2):406-413. doi: 10.1093/brain/awad342. Brain. 2024. PMID: 37796028 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

