Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial
- PMID: 34783839
- PMCID: PMC8596197
- DOI: 10.1001/jama.2021.18463
Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial
Abstract
Importance: There is limited evidence on the benefits of sacubitril/valsartan vs broader renin angiotensin system inhibitor background therapy on surrogate outcome markers, 6-minute walk distance, and quality of life in patients with heart failure and mildly reduced or preserved left ventricular ejection fraction (LVEF >40%).
Objective: To evaluate the effect of sacubitril/valsartan on N-terminal pro-brain natriuretic peptide (NT-proBNP) levels, 6-minute walk distance, and quality of life vs background medication-based individualized comparators in patients with chronic heart failure and LVEF of more than 40%.
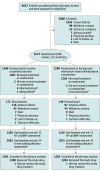
Design, setting, and participants: A 24-week, randomized, double-blind, parallel group clinical trial (August 2017-October 2019). Of 4632 patients screened at 396 centers in 32 countries, 2572 patients with heart failure, LVEF of more than 40%, elevated NT-proBNP levels, structural heart disease, and reduced quality of life were enrolled (last follow-up, October 28, 2019).
Interventions: Patients were randomized 1:1 either to sacubitril/valsartan (n = 1286) or to background medication-based individualized comparator (n = 1286), ie, enalapril, valsartan, or placebo stratified by prior use of a renin angiotensin system inhibitor.
Main outcomes and measures: Primary end points were change from baseline in plasma NT-proBNP level at week 12 and in the 6-minute walk distance at week 24. Secondary end points were change from baseline in quality of life measures and New York Heart Association (NYHA) class at 24 weeks.
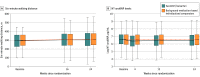
Results: Among 2572 randomized patients (mean age, 72.6 years [SD, 8.5 years]; 1301 women [50.7%]), 2240 (87.1%) completed the trial. At baseline, the median NT-proBNP levels were 786 pg/mL in the sacubitril/valsartan group and 760 pg/mL in the comparator group. After 12 weeks, patients in the sacubitril/valsartan group (adjusted geometric mean ratio to baseline, 0.82 pg/mL) had a significantly greater reduction in NT-proBNP levels than did those in the comparator group (adjusted geometric mean ratio to baseline, 0.98 pg/mL) with an adjusted geometric mean ratio of 0.84 (95% CI, 0.80 to 0.88; P < .001). At week 24, there was no significant between-group difference in median change from baseline in the 6-minute walk distance with an increase of 9.7 m vs 12.2 m (adjusted mean difference, -2.5 m; 95% CI, -8.5 to 3.5; P = .42). There was no significant between-group difference in the mean change in the Kansas City Cardiomyopathy Questionnaire clinical summary score (12.3 vs 11.8; mean difference, 0.52; 95% CI, -0.93 to 1.97) or improvement in NYHA class (23.6% vs 24.0% of patients; adjusted odds ratio, 0.98; 95% CI, 0.81 to 1.18). The most frequent adverse events in the sacubitril/valsartan group vs the comparator group were hypotension (14.1% vs 5.5%), albuminuria (12.3% vs 7.6%), and hyperkalemia (11.6% vs 10.9%).
Conclusions and relevance: Among patients with heart failure and left ventricular ejection factor of higher than 40%, sacubitril/valsartan treatment compared with standard renin angiotensin system inhibitor treatment or placebo resulted in a significantly greater decrease in plasma N-terminal pro-brain natriuretic peptide levels at 12 weeks but did not significantly improve 6-minute walk distance at 24 weeks. Further research is warranted to evaluate potential clinical benefits of sacubitril/valsartan in these patients.
Trial registration: ClinicalTrials.gov Identifier: NCT03066804.
Conflict of interest statement
Figures

Comment in
-
Quality of Life and Exercise Ability in Heart Failure With Preserved Ejection Fraction: No Time for Therapeutic Complacency.JAMA. 2021 Nov 16;326(19):1913-1915. doi: 10.1001/jama.2021.15874. JAMA. 2021. PMID: 34783856 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

