High-throughput single-cell epigenomic profiling by targeted insertion of promoters (TIP-seq)
- PMID: 34783858
- PMCID: PMC8600797
- DOI: 10.1083/jcb.202103078
High-throughput single-cell epigenomic profiling by targeted insertion of promoters (TIP-seq)
Abstract
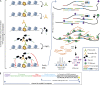
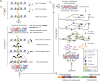
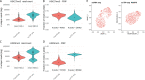
Chromatin profiling in single cells has been extremely challenging and almost exclusively limited to histone proteins. In cases where single-cell methods have shown promise, many require highly specialized equipment or cell type-specific protocols and are relatively low throughput. Here, we combine the advantages of tagmentation, linear amplification, and combinatorial indexing to produce a high-throughput single-cell DNA binding site mapping method that is simple, inexpensive, and capable of multiplexing several independent samples per experiment. Targeted insertion of promoters sequencing (TIP-seq) uses Tn5 fused to proteinA to insert a T7 RNA polymerase promoter adjacent to a chromatin protein of interest. Linear amplification of flanking DNA with T7 polymerase before sequencing library preparation provides ∼10-fold higher unique reads per single cell compared with other methods. We applied TIP-seq to map histone modifications, RNA polymerase II (RNAPII), and transcription factor CTCF binding sites in single human and mouse cells.
© 2021 Bartlett et al.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

