Safety and efficacy of nivolumab plus ipilimumab in patients with advanced renal cell carcinoma with brain metastases: CheckMate 920
- PMID: 34784056
- PMCID: PMC9298991
- DOI: 10.1002/cncr.34016
Safety and efficacy of nivolumab plus ipilimumab in patients with advanced renal cell carcinoma with brain metastases: CheckMate 920
Abstract
Background: Nivolumab plus ipilimumab (NIVO + IPI) has demonstrated long-term efficacy and safety in patients with previously untreated, advanced renal cell carcinoma (aRCC). Although most phase 3 clinical trials exclude patients with brain metastases, the ongoing, multicohort phase 3b/4 CheckMate 920 trial (ClincalTrials.gov identifier NCT02982954) evaluated the safety and efficacy of NIVO + IPI in a cohort that included patients with aRCC and brain metastases, as reported here.
Methods: Patients with previously untreated aRCC and asymptomatic brain metastases received NIVO 3 mg/kg plus IPI 1 mg/kg every 3 weeks × 4 followed by NIVO 480 mg every 4 weeks. The primary end point was the incidence of grade ≥3 immune-mediated adverse events (imAEs) within 100 days of the last dose of study drug. Key secondary end points were progression-free survival and the objective response rate according to Response Evaluation Criteria in Solid Tumors, version 1.1 (both determined by the investigator). Exploratory end points included overall survival, among others.
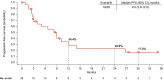
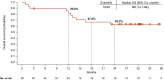
Results: After a minimum follow-up of 24.5 months (N = 28), no grade 5 imAEs occurred. The most common grade 3 and 4 imAEs were diarrhea/colitis (n = 2; 7%) and hypophysitis, rash, hepatitis, and diabetes mellitus (n = 1 each; 4%). The objective response rate was 32% (95% CI, 14.9%-53.5%) with a median duration of response of 24.0 months; 4 of 8 responders remained without reported progression. Seven patients (25%) had intracranial progression. The median progression-free survival was 9.0 months (95% CI, 2.9-12.0 months), and the median overall survival was not reached (95% CI, 14.1 months to not estimable).
Conclusions: In patients who had previously untreated aRCC and brain metastases-a population with a high unmet medical need that often is underrepresented in clinical trials-the approved regimen of NIVO + IPI followed by NIVO showed encouraging antitumor activity and no new safety signals.
Keywords: aRCC; brain metastases; intracranial; ipilimumab; nivolumab; renal cell carcinoma; unmet need.
© 2021 The Authors. Cancer published by Wiley Periodicals LLC on behalf of American Cancer Society.
Conflict of interest statement
Hamid Emamekhoo reports funding from Bristol Myers Squibb (BMS) for the article during the conduct of the study; and personal fees from Exelixis, Bayer, BMS, and Cardinal Health; travel support from DAVA Oncology; and institutional research funding from BMS outside the submitted work. Alexandra Drakaki reports research funding from Kite/Gilead; institutional research funding from AstraZeneca, Genentech/Roche, BMS, Merck Sharp & Dohme, Jounce Therapeutics, Infinity Pharmaceuticals, and Seattle Genetics/Astellas; personal fees from BMS, AstraZeneca, Radmetrix, Seattle Genetics, Janssen, PACT Pharma, Merck, Roche/Genentech, Exelixis, and Dyania Health; grants or contracts from A2, Merck, Genentech, Exelixis, and Seattle Genetics; and travel support from Lilly, AstraZeneca, and Seattle Genetics outside the submitted work. Ana M. Molina reports personal fees from Exelixis, Janssen, and Eisai and honoraria from the American Society of Clinical Oncology outside the submitted work. Daniel C. Cho reports personal fees from Amphivena, HUYA Bioscience International, Nektar Therapeutics, Pfizer, and Werewolf; payment for expert testimony from AbbVie and Genentech; and participation on a Nektar Therapeutics advisory board outside the submitted work. Johanna C. Bendell reports institutional consulting/advisory fees from Gilead Sciences, Genentech/Roche, BMS, Five Prime Therapeutics, Lilly, Merck, MedImmune, Celgene, EMD Serono, Taiho Pharmaceutical, MacroGenics, GlaxoSmithKline, Novartis, OncoMed, Leap Therapeutics, TG Therapeutics, AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, Bayer, Incyte, Apexigen, Array BioPharma, Sanofi, Ipsen Merrimack, OncoGenex, FORMA Therapeutics, Arch Oncology, Prelude Therapeutics, Phoenix Biotech, Cyteir, Molecular Partners, Innate Pharma, Torque, Tizona Therapeutics, Janssen, Tolero Pharmaceuticals, TD2, Amgen, Seattle Genetics, Moderna Therapeutics, Tanabe Research, BeiGene, Continuum Clinical, Cerulean Pharma, Kyn Therapeutics, Bicycle Therapeutics, Relay Therapeutics, Evelo Therapeutics, and Fusion Pharmaceuticals; travel support from Merck, Roche/Genentech, Celgene, Daiichi Sankyo, Gilead Sciences, BMS, Lilly, MedImmune, Taiho Pharmaceutical, Novartis, OncoMed, Boehringer Ingelheim, ARMO Biosciences, Ipsen, and FORMA Therapeutics; and institutional research funding from Lilly, Genentech/Roche, Incyte, Gilead Sciences, BMS, Leap Therapeutics, AstraZeneca/MedImmune, Boston Biomedical, GlaxoSmithKline, Novartis, Array BioPharma, Taiho Pharmaceutical, Celgene, OncoMed, Daiichi Sankyo, Bayer, Apexigen, Kolltan Pharmaceuticals, SynDevRx, Merck, MacroGenics, Five Prime Therapeutics, EMD Serono, TG Therapeutics, Boehringer Ingelheim, Forty Seven, Stemcentrx, Onyx, Sanofi, Takeda, Abbott/AbbVie, Eisai, Celldex, Agios, ARMO Biosciences, CytomX Therapeutics, Nektar Therapeutics, Ipsen, Merrimack, Tarveda Therapeutics, Tyrogenex, OncoGenex, Marshall Edwards, Pieris Pharmaceuticals, Mersana, Calithera Biosciences, Blueprint Medicines, Gritstone Oncology, Evelo Therapeutics, Forma Therapeutics, Merus, Jacobio, eFFECTOR Therapeutics, Novocure, Sorrento Therapeutics, Arrys Therapeutics, TRACON Pharma, Sierra Oncology, Prelude Therapeutics, Arch Oncology, Harpoon Therapeutics, Phoenix Biotech, Unum Therapeutics, Vyriad, Cyteir, Molecular Partners, ADC Therapeutics, Torque, Tizona Therapeutics, Janssen, Amgen, BeiGene, Pfizer, Millennium Pharmaceuticals, ImClone Systems, Acerta Pharma, Rgenix, Bellicum Pharmaceuticals, Arcus Biosciences, Gossamer Bio, Seattle Genetics, Tempest Therapeutics, Synthorx, Revolution Medicines, Bicycle Therapeutics, ZymeWorks, Relay Therapeutics, Scholar Rock, NGM Biopharmaceuticals, Numab, AtlasMedx, Treadwell Therapeutics, IGM, MabSpace Biosciences, Hutchison MediPharma, Repare Therapeutics, NeoImmuneTech, Regeneron, PureTech, G1 Therapeutics, Erasca Inc, Rubius Therapeutics, Pionyr, Loxo/Lilly, and BioNTech AG all outside the submitted work. Lucio N. Gordan is an employee of Florida Cancer Specialists with a leadership role and reports honoraria from Ameris Pharma outside the submitted work. Arash Rezazadeh Kalebasty reports grants from BMS during the conduct of the study; and personal fees from Exelixis, AstraZeneca, Bayer, BMS, Pfizer, Novartis, Genentech, Gilead Sciences, and EMD Serono; honoraria from Janssen, Astellas Medivation, Pfizer, Novartis, Sanofi, Genentech/Roche, Eisai, AstraZeneca, BMS, Amgen, Exelixis, EMD Serono, Merck, Seattle Genetics/Astellas, Myovant Sciences, and AVEO; meeting/travel support from Genentech, Prometheus, Astellas Medivation, Janssen, Eisai, Bayer, Pfizer, Novartis, Exelixis, AstraZeneca, and ECOM Medical; and institutional research funding from Genentech, Exelixis, Janssen, AstraZeneca, Bayer, BMS, Eisai, MacroGenics, Astellas Pharma, BeyondSpring Pharmaceuticals, BioClin Therapeutics, Clovis Oncology, Bavarian Nordic, Seattle Genetics, Immunomedics, and Epizyme outside the submitted work. Daniel J. George reports institutional grants from Astellas Pharma, AstraZeneca, BMS, Calithera Biosciences, Exelixis, Janssen Oncology, Novartis, Pfizer, and Sanofi/Aventis; personal fees from Bayer, Exelixis, Pfizer, Sanofi, Astellas Pharma, BMS, Genentech, Janssen, Merck Sharp & Dohme, Myovant Sciences, AstraZeneca, Michael J. Hennessy Associates, Propella Therapeutics (formerly Vizuri), Constellation Pharmaceuticals, Flatiron, Modra Pharmaceuticals, Physician Education Resource, and RevHealth, LLC; honoraria/speakers fees from Sanofi, Bayer, Exelixis, EMD Serono, OncLive, Pfizer, UroToday, the American Association for Cancer Research, Axess Oncology, Janssen Oncology, Millennium Medical Publication, Ipsen, and UroGPO; meeting/travel support from Bayer, Exelixis, Sanofi, and UroToday; and participation on advisory boards for Astellas, AstraZeneca, Capio Biosciences, Janssen Oncology, and Modra Pharma outside the submitted work. Thomas E. Hutson is an employee of Texas Oncology; he reports personal fees from Astellas Pharma, AstraZeneca, AVEO, Bayer/Onyx, BMS, Eisai, Exelixis, Janssen, Johnson & Johnson, Novartis, and Pfizer; speakers fees from Pfizer, Johnson & Johnson, Eisai, Exelixis, Astellas Pharma, and BMS; honoraria from Pfizer, Astellas Pharma, BMS, Exelixis, Eisai, Novartis, Johnson & Johnson, and Bayer/Onyx; and institutional research funding from Pfizer, Johnson & Johnson, Exelixis, Eisai, and BMS outside the submitted work. Edward R. Arrowsmith is an employee of Tennessee Oncology; he reports travel support from Flatiron Health, OneOncology, and the Sarah Cannon Research Institute; personal fees from the Sarah Cannon Research Institute; stock ownership in OneOncology; and institutional research funding from AstraZeneca, Boehringer Ingelheim, BMS, Calistoga Pharmaceuticals, Celgene, Cephalon, Chorus, Cougar Biotechnology, Eisai, EMD Serono, Evelo Biosciences, Exelixis, Genentech, Gilead Sciences, Incyte, Merck, Millennium, Modra Pharmaceuticals, Novartis, OncoGenex, Onyx, Peloton Therapeutics, Pfizer, the Sarah Cannon Research Institute, Takeda, Clovis Oncology, Lilly, Infinity Pharmaceuticals, and Janssen Research & Development all outside the submitted work. Joshua Zhang is an employee of BMS and owns stock in the company. Jesus Zoco is an employee of Syneos Health and reports personal fees from Syneos Health outside the submitted work. Jennifer L. Johansen is an employee of BMS and owns stock in the company. David K. Leung is an employee of BMS and owns stock in the company; he reports travel support from BMS and holds institutional patents with BMS. Scott S. Tykodi reports support from BMS for medical writing during the conduct of the study; and personal fees from Merck, Intellisphere LLC, Natera, BMS, Exelixis, and PLS Group Services; honoraria from Natera and General Dynamics Information Technology; and institutional research funding from Genentech, BMS, Merck Sharp & Dohme, Calithera Bioscience, Pfizer, Jounce Therapeutics, Nektar Therapeutics, Exelixis, and the Clinigen Group outside the submitted work. Mark R. Olsen and Ivor J. Percent made no disclosures.
Figures
Comment in
-
Entering the endgame for advanced RCC?Nat Rev Urol. 2022 Jan;19(1):4. doi: 10.1038/s41585-021-00547-0. Nat Rev Urol. 2022. PMID: 34845347 No abstract available.
References
-
- National Cancer Institute; Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Kidney and Renal Pelvis Cancer. Accessed March 26, 2021. https://seer.cancer.gov/statfacts/html/kidrp.html
-
- Kattan J, Rassy EE, Assi T, Bakouny Z, Pavlidis N. A comprehensive review of the role of immune checkpoint inhibitors in brain metastasis of renal cell carcinoma origin. Crit Rev Oncol Hematol. 2018;130:60‐69. - PubMed