Anode Material Options Toward 500 Wh kg-1 Lithium-Sulfur Batteries
- PMID: 34784102
- PMCID: PMC8805573
- DOI: 10.1002/advs.202103910
Anode Material Options Toward 500 Wh kg-1 Lithium-Sulfur Batteries
Abstract
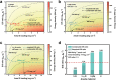
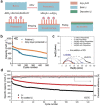
Lithium-sulfur (Li-S) battery is identified as one of the most promising next-generation energy storage systems due to its ultra-high theoretical energy density up to 2600 Wh kg-1 . However, Li metal anode suffers from dramatic volume change during cycling, continuous corrosion by polysulfide electrolyte, and dendrite formation, rendering limited cycling lifespan. Considering Li metal anode as a double-edged sword that contributes to ultrahigh energy density as well as limited cycling lifespan, it is necessary to evaluate Li-based alloy as anode materials to substitute Li metal for high-performance Li-S batteries. In this contribution, the authors systematically evaluate the potential and feasibility of using Li metal or Li-based alloys to construct Li-S batteries with an actual energy density of 500 Wh kg-1 . A quantitative analysis method is proposed by evaluating the required amount of electrolyte for a targeted energy density. Based on a three-level (ideal material level, practical electrode level, and pouch cell level) analysis, highly lithiated lithium-magnesium (Li-Mg) alloy is capable to achieve 500 Wh kg-1 Li-S batteries besides Li metal. Accordingly, research on Li-Mg and other Li-based alloys are reviewed to inspire a promising pathway to realize high-energy-density and long-cycling Li-S batteries.
Keywords: high energy density; lithium metal anodes; lithium-magnesium alloys; lithium-sulfur batteries; pouch cells.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Deciphering the Species-Dependent Polysulfide Corrosion on Lithium Anode Toward Durable Lithium-Sulfur Batteries.Adv Mater. 2025 Jul 1:e2506132. doi: 10.1002/adma.202506132. Online ahead of print. Adv Mater. 2025. PMID: 40590145
-
Correlating Polysulfide Solvation Structure with Electrode Kinetics towards Long-Cycling Lithium-Sulfur Batteries.Angew Chem Int Ed Engl. 2023 Oct 23;62(43):e202309968. doi: 10.1002/anie.202309968. Epub 2023 Sep 15. Angew Chem Int Ed Engl. 2023. PMID: 37664907
-
Electrolyte Regulation towards Stable Lithium-Metal Anodes in Lithium-Sulfur Batteries with Sulfurized Polyacrylonitrile Cathodes.Angew Chem Int Ed Engl. 2020 Jun 26;59(27):10732-10745. doi: 10.1002/anie.201912701. Epub 2020 Apr 1. Angew Chem Int Ed Engl. 2020. PMID: 31746521 Review.
-
An Organodiselenide Comediator to Facilitate Sulfur Redox Kinetics in Lithium-Sulfur Batteries with Encapsulating Lithium Polysulfide Electrolyte.Angew Chem Int Ed Engl. 2023 Jul 24;62(30):e202303363. doi: 10.1002/anie.202303363. Epub 2023 Jun 20. Angew Chem Int Ed Engl. 2023. PMID: 37249483
-
Anode Improvement in Rechargeable Lithium-Sulfur Batteries.Adv Mater. 2017 Dec;29(48). doi: 10.1002/adma.201700542. Epub 2017 Jun 19. Adv Mater. 2017. PMID: 28626966 Review.
Cited by
-
Atomic and Molecular Layer Deposition as Surface Engineering Techniques for Emerging Alkali Metal Rechargeable Batteries.Molecules. 2022 Sep 20;27(19):6170. doi: 10.3390/molecules27196170. Molecules. 2022. PMID: 36234705 Free PMC article. Review.
-
Li, Na, K, Mg, Zn, Al, and Ca Anode Interface Chemistries Developed by Solid-State Electrolytes.Adv Sci (Weinh). 2023 Nov;10(32):e2304235. doi: 10.1002/advs.202304235. Epub 2023 Sep 24. Adv Sci (Weinh). 2023. PMID: 37743719 Free PMC article. Review.
-
Chlorine bridge bond-enabled binuclear copper complex for electrocatalyzing lithium-sulfur reactions.Nat Commun. 2024 Apr 15;15(1):3231. doi: 10.1038/s41467-024-47565-1. Nat Commun. 2024. PMID: 38622167 Free PMC article.
-
Versatile Asymmetric Separator with Dendrite-Free Alloy Anode Enables High-Performance Li-S Batteries.Adv Sci (Weinh). 2022 Sep;9(25):e2202204. doi: 10.1002/advs.202202204. Epub 2022 Jun 24. Adv Sci (Weinh). 2022. PMID: 35748192 Free PMC article.
-
Fluorinated Multi-Walled Carbon Nanotubes Coated Separator Mitigates Polysulfide Shuttle in Lithium-Sulfur Batteries.Materials (Basel). 2023 Feb 22;16(5):1804. doi: 10.3390/ma16051804. Materials (Basel). 2023. PMID: 36902922 Free PMC article.
References
-
- a) Gur T. M., Energy Environ. Sci. 2018, 11, 2696;
- b) Shen X., Zhang X.‐Q., Ding F., Huang J.‐Q., Xu R., Chen X., Yan C., Su F.‐Y., Chen C.‐M., Liu X., Zhang Q., Energy Mater. Adv. 2021, 2021, 1205324; - PubMed
- c) Kong L., Tang C., Peng H.‐J., Huang J.‐Q., Zhang Q., SmartMat 2020, 1, e1007.
-
- a) Ding Y., Cano Z. P., Yu A., Lu J., Chen Z., Electrochem. Energy Rev. 2019, 2, 1;<./bib>
- b) Dunn B., Kamath H., Tarascon J. M., Science 2011, 334, 928. - PubMed
-
- Nitta N., Wu F., Lee J. T., Yushin G., Mater. Today 2015, 18, 252.
-
- Liu J., Bao Z., Cui Y., Dufek E. J., Goodenough J. B., Khalifah P., Li Q., Liaw B. Y., Liu P., Manthiram A., Meng Y. S., Subramanian V. R., Toney M. F., Viswanathan V. V., Whittingham M. S., Xiao J., Xu W., Yang J., Yang X.‐Q., Zhang J.‐G., Nat. Energy 2019, 4, 180.
-
- a) Dörfler S., Althues H., Härtel P., Abendroth T., Schumm B., Kaskel S., Joule 2020, 4, 539;
- b) Peng H. J., Huang J. Q., Zhang Q., Chem. Soc. Rev. 2017, 46, 5237. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
