Synthesis of Bis-heteroaryls Using Grignard Reagents and Pyridylsulfonium Salts
- PMID: 34784224
- PMCID: PMC8650099
- DOI: 10.1021/acs.orglett.1c03379
Synthesis of Bis-heteroaryls Using Grignard Reagents and Pyridylsulfonium Salts
Abstract
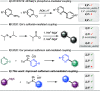
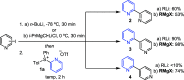
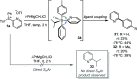
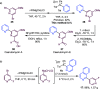
Herein are reported ligand-coupling reactions of Grignard reagents with pyridylsulfonium salts. The method has wide functional group tolerance and enables the formation of bis-heterocycle linkages, including 2,4'-, 2,3'-, and 2,2'-bipyridines, as well as pyridines linked to pyrimidines, pyrazines, isoxazoles, and benzothiophenes. The methodology was successfully applied to the synthesis of the natural products caerulomycin A and E.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Synthesis of Pyridylsulfonium Salts and Their Application in the Formation of Functionalized Bipyridines.Org Lett. 2020 Nov 6;22(21):8451-8457. doi: 10.1021/acs.orglett.0c03048. Epub 2020 Oct 22. Org Lett. 2020. PMID: 33090810
-
Copper-catalysed cross-coupling of alkyl Grignard reagents and propargylic ammonium salts: stereospecific synthesis of allenes.Chem Commun (Camb). 2018 Jul 24;54(60):8343-8346. doi: 10.1039/c8cc03760d. Chem Commun (Camb). 2018. PMID: 29992220
-
Sequential one-pot addition of excess aryl-Grignard reagents and electrophiles to O-alkyl thioformates.Chemistry. 2013 Sep 23;19(39):13112-9. doi: 10.1002/chem.201301573. Epub 2013 Aug 14. Chemistry. 2013. PMID: 23946145
-
Catalytic highly enantioselective alkylation of aldehydes with deactivated grignard reagents and synthesis of bioactive intermediate secondary arylpropanols.J Org Chem. 2010 Oct 15;75(20):6869-78. doi: 10.1021/jo101351t. J Org Chem. 2010. PMID: 20836546
-
Heavy Grignard Reagents: Synthesis, Physical and Structural Properties, Chemical Behavior, and Reactivity.Chemistry. 2017 Jan 31;23(7):1456-1483. doi: 10.1002/chem.201603786. Epub 2016 Dec 15. Chemistry. 2017. PMID: 27976821 Review.
Cited by
-
Electrochemical synthesis of biaryls by reductive extrusion from N,N'-diarylureas.Nat Commun. 2023 Jul 28;14(1):4561. doi: 10.1038/s41467-023-40237-6. Nat Commun. 2023. PMID: 37507363 Free PMC article.
-
First total synthesis of caerulomycin K: a case study on selective, multiple C-H functionalizations of pyridines.RSC Adv. 2024 Feb 13;14(8):5542-5546. doi: 10.1039/d4ra00589a. eCollection 2024 Feb 7. RSC Adv. 2024. PMID: 38352680 Free PMC article.
References
-
- Oae S.; Uchida Y. Ligand-Coupling Reactions of Hypervalent Species. Acc. Chem. Res. 1991, 24 (7), 202–208. 10.1021/ar00007a003. - DOI
-
- Oae S.; Inubushi Y.; Yoshihara M. Thionyl Chloride-a Good Ligand Coupling Reagent. Phosphorus, Sulfur Silicon Relat. Elem. 1995, 103 (1–4), 101–110. 10.1080/10426509508027369. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

