PD-1 blockade counteracts post-COVID-19 immune abnormalities and stimulates the anti-SARS-CoV-2 immune response
- PMID: 34784300
- PMCID: PMC8783674
- DOI: 10.1172/jci.insight.146701
PD-1 blockade counteracts post-COVID-19 immune abnormalities and stimulates the anti-SARS-CoV-2 immune response
Abstract
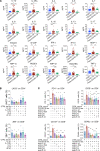
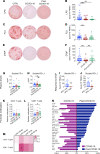
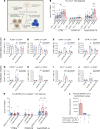
A substantial proportion of patients who have recovered from coronavirus disease-2019 (COVID-19) experience COVID-19-related symptoms even months after hospital discharge. We extensively immunologically characterized patients who recovered from COVID-19. In these patients, T cells were exhausted, with increased PD-1+ T cells, as compared with healthy controls. Plasma levels of IL-1β, IL-1RA, and IL-8, among others, were also increased in patients who recovered from COVID-19. This altered immunophenotype was mirrored by a reduced ex vivo T cell response to both nonspecific and specific stimulation, revealing a dysfunctional status of T cells, including a poor response to SARS-CoV-2 antigens. Altered levels of plasma soluble PD-L1, as well as of PD1 promoter methylation and PD1-targeting miR-15-5p, in CD8+ T cells were also observed, suggesting abnormal function of the PD-1/PD-L1 immune checkpoint axis. Notably, ex vivo blockade of PD-1 nearly normalized the aforementioned immunophenotype and restored T cell function, reverting the observed post-COVID-19 immune abnormalities; indeed, we also noted an increased T cell-mediated response to SARS-CoV-2 peptides. Finally, in a neutralization assay, PD-1 blockade did not alter the ability of T cells to neutralize SARS-CoV-2 spike pseudotyped lentivirus infection. Immune checkpoint blockade ameliorates post-COVID-19 immune abnormalities and stimulates an anti-SARS-CoV-2 immune response.
Keywords: Anergy; COVID-19; Immunology; Immunotherapy.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

