Efficacy and safety of salmeterol/fluticasone compared with montelukast alone (or add-on therapy to fluticasone) in the treatment of bronchial asthma in children and adolescents: a systematic review and meta-analysis
- PMID: 34784306
- PMCID: PMC8710318
- DOI: 10.1097/CM9.0000000000001853
Efficacy and safety of salmeterol/fluticasone compared with montelukast alone (or add-on therapy to fluticasone) in the treatment of bronchial asthma in children and adolescents: a systematic review and meta-analysis
Abstract
Background: Despite the recommendation of inhaled corticosteroids (ICSs) plus long-acting beta 2-agonist (LABA) and leukotriene receptor antagonist (LTRA) or ICS/LTRA as stepwise approaches in asthmatic children, there is a lack of published systematic review comparing the efficacy and safety of the two therapies in children and adolescents aged 4 to 18 years. This study aimed to compare the safety and efficacy of salmeterol/fluticasone (SFC) vs. montelukast (MON), or combination of montelukast and fluticasone (MFC) in children and adolescents aged 4 to 18 years with bronchial asthma.
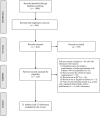
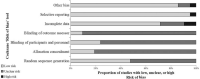
Methods: A systematic search was conducted in MEDLINE, EMBASE, the Cochrane Library, China BioMedical Literature Database, Chinese National Knowledge Infrastructure, VIP Database for Chinese Technical Periodical, and Wanfang for randomized controlled trials (RCTs) published from inception to May 24, 2021. Interventions are as follows: SFC vs. MON, or combination of MFC, with no limitation of dosage or duration. Primary and secondary outcome measures were as follows: the primary outcome of interest was the risk of asthma exacerbation. Secondary outcomes included risk of hospitalization, pulmonary function, asthma control level, quality of life, and adverse events (AEs). A random-effects (I2 ≥ 50%) or fixed-effects model (I2 < 50%) was used to calculate pooled effect estimates, comparing the outcomes between the intervention and control groups where feasible.
Results: Of the 1006 articles identified, 21 studies met the inclusion criteria with 2643 individuals; two were at low risk of bias. As no primary outcomes were similar after an identical treatment duration in the included studies, meta-analysis could not be performed. However, more studies favored SFC, instead of MON, owing to a lower risk of asthma exacerbation in the SFC group. As for secondary outcome, SFC showed a significant improvement of peak expiratory flow (PEF)%pred after 4 weeks compared with MFC (mean difference [MD]: 5.45; 95% confidence interval [CI]: 1.57-9.34; I2 = 95%; P = 0.006). As for asthma control level, SFC also showed a higher full-controlled level (risk ratio [RR]: 1.51; 95% CI: 1.24-1.85; I2 = 0; P < 0.001) and higher childhood asthma control test score after 4 weeks of treatment (MD: 2.30; 95% CI: 1.39-3.21; I2 = 72%; P < 0.001) compared with MFC.
Conclusions: SFC may be more effective than MFC for the treatment of asthma in children and adolescents, especially in improving asthma control level. However, there is insufficient evidence to make firm conclusive statements on the use of SFC or MON in children and adolescents aged 4 to 18 years with asthma. Further research is needed, particularly a combination of good-quality long-term prospective studies and well-designed RCTs.
Prospero registration number: CRD42019133156.
Copyright © 2021 The Chinese Medical Association, produced by Wolters Kluwer, Inc. under the CC-BY-NC-ND license.
Conflict of interest statement
This work was supported by GlaxoSmithKline (GSK), China. The company did not participant in data screening, extraction and analysis.
Figures
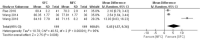
Similar articles
-
Efficacy and tolerability of salmeterol/fluticasone propionate versus montelukast in childhood asthma: A prospective, randomized, double-blind, double-dummy, parallel-group study.Clin Ther. 2008 Aug;30(8):1492-504. doi: 10.1016/j.clinthera.2008.07.018. Clin Ther. 2008. PMID: 18803991 Clinical Trial.
-
Management of Asthma in School age Children On Therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety.Health Technol Assess. 2013 Feb;17(4):1-218. doi: 10.3310/hta17040. Health Technol Assess. 2013. PMID: 23380178 Free PMC article. Clinical Trial.
-
Administrative claims analysis of asthma-related health care utilization for patients who received inhaled corticosteroids with either montelukast or salmeterol as combination therapy.J Manag Care Pharm. 2006 May;12(4):310-21. doi: 10.18553/jmcp.2006.12.4.310. J Manag Care Pharm. 2006. PMID: 16792437 Free PMC article.
-
Inhaled salmeterol/fluticasone propionate combination: a review of its use in persistent asthma.Drugs. 2000 Nov;60(5):1207-33. doi: 10.2165/00003495-200060050-00012. Drugs. 2000. PMID: 11129128 Review.
-
Treatment options for initial maintenance therapy of persistent asthma: a review of inhaled corticosteroids and leukotriene receptor antagonists.Drugs. 2003;63 Suppl 2:1-20. doi: 10.2165/00003495-200363002-00002. Drugs. 2003. PMID: 14984077 Review.
Cited by
-
Maintenance Therapy for Children and Adolescents with Asthma: Guidelines and Recommendations from the Emilia-Romagna Asthma (ERA) Study Group.J Clin Med. 2023 Aug 23;12(17):5467. doi: 10.3390/jcm12175467. J Clin Med. 2023. PMID: 37685533 Free PMC article.
-
Determining the relationship between the knowledge on self-management and levels of asthma control among adult asthmatic patients: a cross-sectional study.J Med Life. 2023 Mar;16(3):442-446. doi: 10.25122/jml-2022-0333. J Med Life. 2023. PMID: 37168308 Free PMC article.
-
Green spaces exposure and the risk of common psychiatric disorders: A meta-analysis.SSM Popul Health. 2024 Feb 15;25:101630. doi: 10.1016/j.ssmph.2024.101630. eCollection 2024 Mar. SSM Popul Health. 2024. PMID: 38405164 Free PMC article.
-
A Bibliometric Analysis of Medication Compliance in Children with Asthma.J Multidiscip Healthc. 2025 May 23;18:2875-2887. doi: 10.2147/JMDH.S514612. eCollection 2025. J Multidiscip Healthc. 2025. PMID: 40433421 Free PMC article.
-
Achieving remission in severe asthma.Chin Med J Pulm Crit Care Med. 2025 Jun 13;3(2):77-87. doi: 10.1016/j.pccm.2025.05.001. eCollection 2025 Jun. Chin Med J Pulm Crit Care Med. 2025. PMID: 40677418 Free PMC article. Review.
References
-
- Zhao J. National parents of asthmatic children KAP project team. Asthma control status in children and related factors in 29 cities of China (in Chinese). Chin J Pediatr 2013; 51:90–95. doi: 10.3760/cma.j.issn.0578-1310.2013.02.003. - PubMed
-
- Sha L, Liu C, Shao M, Chen Y. Ten years comparison of diagnosis and treatment of asthma in urban children in China (in Chinese). Chin J Pediatr 2016; 54:182–186. doi: 10.3760/cma.j.issn.0578-1310.2016.03.005. - PubMed
-
- Zhou X, Hong J. Pediatric asthma management in China: current and future challenges. Pediatr Drugs 2018; 20:105–110. doi: 10.1007/s40272-017-0276-7. - PubMed
-
- Xiang L, Zhao J, Zheng Y, Liu H, Hong J, Bao Y, et al. . Uncontrolled asthma and its risk factors in Chinese children: a cross-sectional observational study. J Asthma 2016; 53:699–706. doi: 10.3109/02770903.2016.1144199. - PubMed
-
- Bao Y, Chen A, Fu Z, Li C, Liu C, Xiang L, et al. . Guidelines for the diagnosis and management of bronchial asthma in children (2016 edition) (in Chinese). Chin J Pediatr 2016; 3:167–181. doi: 10.3321/j.issn:0578-1310.2008.10.006.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

