Serologic titers to Leptospira in vaccinated pigs and interpretation for surveillance
- PMID: 34784395
- PMCID: PMC8594815
- DOI: 10.1371/journal.pone.0260052
Serologic titers to Leptospira in vaccinated pigs and interpretation for surveillance
Abstract
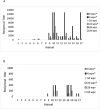
Diagnosis and surveillance of pathogenic Leptospira is difficult as organisms may be intermittently shed and in small numbers. Therefore, serologic testing by the microscopic agglutination test (MAT) is the primary screening method for leptospirosis. While a MAT titer ≥1:100 is considered to be a positive result, interpretation is complicated by the use of commercial vaccines in pigs. Most guidelines for interpretation of MAT titers in pigs were published in the 1970's and 1980's, prior to the development of the current multivalent vaccines. We evaluated MAT titers in routinely vaccinated healthy research pigs compared to their unvaccinated cohorts. Our study confirmed previous reports that the Pomona serovar elicits minimal antibody response even after a second booster 6 months after initial vaccination. However, MAT titers of ≥1:3,200 were detected as early as 4 weeks post initial vaccination for serovars Bratislava and Icterohaemorrhagiae and remained as high as ≥1:1,600 prior to booster at 24 weeks post vaccination. Our study determined that high levels of MAT titers can occur from vaccination alone and high titers are not necessarily indicative of infection. Therefore, the interpretation of MAT titers as indicators of Leptospira infection should be readdressed.
Conflict of interest statement
The authors have declared no competing interests exist.
Figures
References
-
- Yasuda PH, Steigerwalt AG, Sulzer KR, Kaufmann AF, Rogers F, Brenner DJ. Deoxyribonucleic Acid Relatedness between Serogroups and Serovars in the Family Leptospiraceae with Proposals for Seven New Leptospira Species. International Journal of Systematic and Evolutionary Microbiology. 1987;37(4):407–15.
-
- Bolin C. Leptospirosis. In: Brown C, Bolin CA, editors. Emerging Diseases of Animals. Washington, D.C.: ASM Press; 2000. p. 185–200.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

