Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial
- PMID: 34784492
- PMCID: PMC8906485
- DOI: 10.1164/rccm.202108-1986OC
Clinical Effectiveness of Elexacaftor/Tezacaftor/Ivacaftor in People with Cystic Fibrosis: A Clinical Trial
Abstract
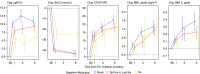
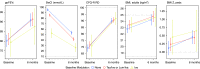
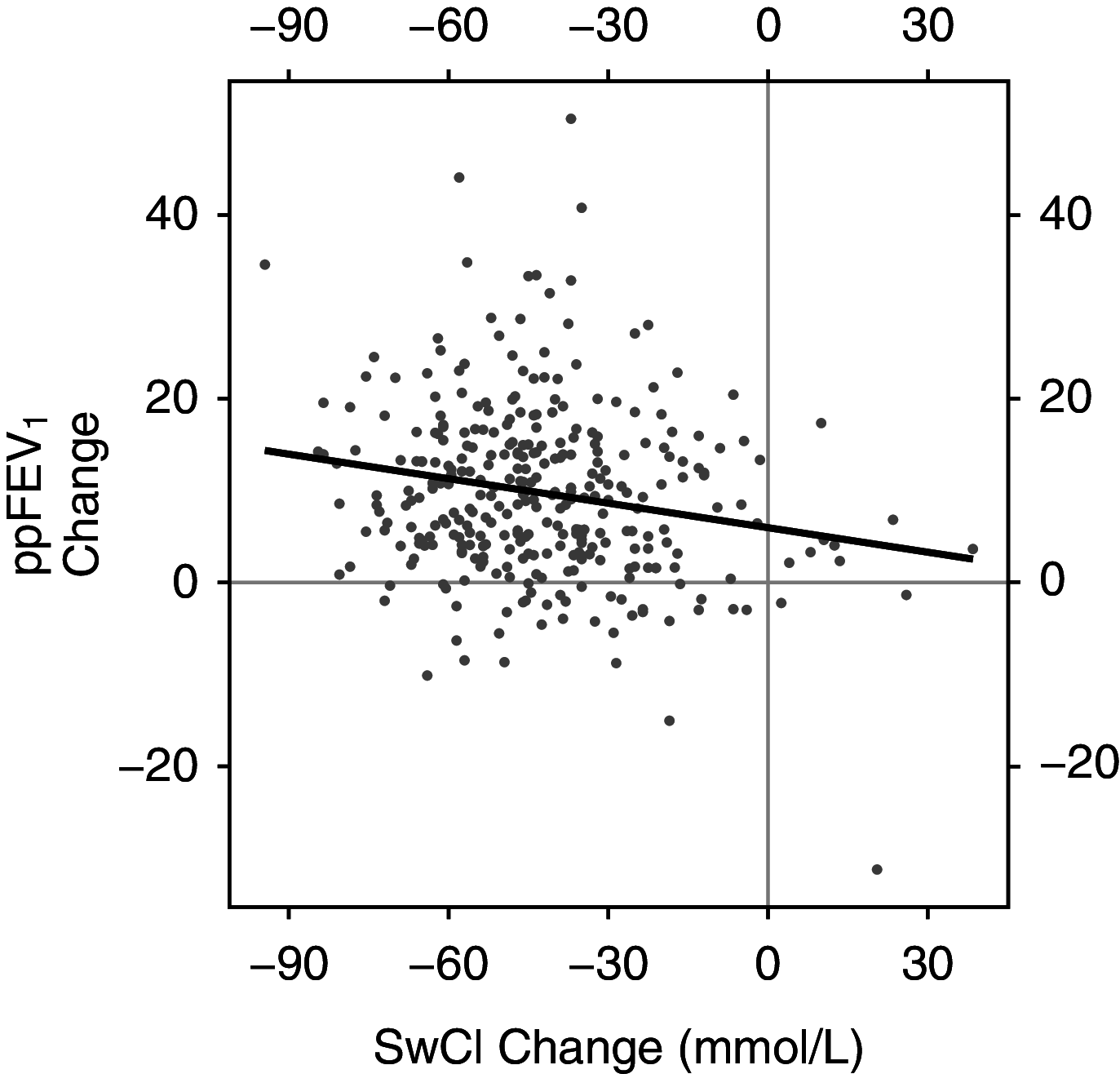
Rationale: The cystic fibrosis (CF) modulator drug, elexacaftor/tezacaftor/ivacaftor (ETI), proved highly effective in controlled clinical trials for individuals with at least one F508del allele, which occurs in at least 85% of people with CF. Objectives: PROMISE is a postapproval study to understand the broad effects of ETI through 30 months' clinical use in a more diverse U.S. patient population with planned analyses after 6 months. Methods: Prospective, observational study in 487 people with CF age 12 years or older with at least one F508del allele starting ETI for the first time. Assessments occurred before and 1, 3, and 6 months into ETI therapy. Outcomes included change in percent predicted FEV1 (ppFEV1), sweat chloride concentration, body mass index (BMI), and self-reported respiratory symptoms. Measurements and Main Results: Average age was 25.1 years, and 44.1% entered the study using tezacaftor/ivacaftor or lumacaftor/ivacaftor, whereas 6.7% were using ivacaftor, consistent with F508del homozygosity and G551D allele, respectively. At 6 months into ETI therapy, ppFEV1 improved 9.76 percentage points (95% confidence interval [CI], 8.76 to 10.76) from baseline, cystic fibrosis questionnaire-revised respiratory domain score improved 20.4 points (95% CI, 18.3 to 22.5), and sweat chloride decreased -41.7 mmol/L (95% CI, -43.8 to -39.6). BMI also significantly increased. Changes were larger in those naive to modulators but substantial in all groups, including those treated with ivacaftor at baseline. Conclusions: ETI by clinical prescription provided large improvements in lung function, respiratory symptoms, and BMI in a diverse population naive to modulator drug therapy, using existing two-drug combinations, or using ivacaftor alone. Each group also experienced significant reductions in sweat chloride concentration, which correlated with improved ppFEV1 in the overall study population. Clinical trial registered with www.clinicaltrials.gov (NCT NCT04038047).
Keywords: PROMISE; clinical trial; cystic fibrosis; elexacaftor/tezacaftor/ivacaftor; modulators.
Figures
Comment in
-
Cystic Fibrosis: A Disease in Transformation, yet More Work to Be Done!Am J Respir Crit Care Med. 2022 Mar 1;205(5):487-489. doi: 10.1164/rccm.202112-2782ED. Am J Respir Crit Care Med. 2022. PMID: 35073504 Free PMC article. No abstract available.
-
Reply to Martin et al.: Change in Lung Function after Initiation of Elexacaftor-Tezacaftor-Ivacaftor: Do Not Forget Anatomy!Am J Respir Crit Care Med. 2022 Jun 1;205(11):1366-1367. doi: 10.1164/rccm.202201-0042LE. Am J Respir Crit Care Med. 2022. PMID: 35358029 Free PMC article. No abstract available.
-
Change in Lung Function after Initiation of Elexacaftor-Tezacaftor-Ivacaftor: Do Not Forget Anatomy!Am J Respir Crit Care Med. 2022 Jun 1;205(11):1365-1366. doi: 10.1164/rccm.202112-2852LE. Am J Respir Crit Care Med. 2022. PMID: 35358030 Free PMC article. No abstract available.
References
-
- Rowe SM, Heltshe SL, Gonska T, Donaldson SH, Borowitz D, Gelfond D, et al. GOAL Investigators of the Cystic Fibrosis Foundation Therapeutics Development Network Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med . 2014;190:175–184. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

