The PREvention Program for Alzheimer's RElated Delirium (PREPARED) cluster randomized trial: a study protocol
- PMID: 34784897
- PMCID: PMC8594158
- DOI: 10.1186/s12877-021-02558-3
The PREvention Program for Alzheimer's RElated Delirium (PREPARED) cluster randomized trial: a study protocol
Abstract
Background: Delirium is a significant cause of morbidity and mortality among older people admitted to both acute and long-term care facilities (LTCFs). Multicomponent interventions have been shown to reduce delirium incidence in the acute care setting (30-73%) by acting on modifiable risk factors. Little work, however, has focused on using this approach to reduce delirium incidence in LTCFs.
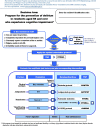
Methods: The objective is to assess the effectiveness of the multicomponent PREPARED Trial intervention in reducing the following primary outcomes: incidence, severity, duration, and frequency of delirium episodes in cognitively impaired residents. This 4-year, parallel-design, cluster randomized study will involve nursing staff and residents in 45-50 LTCFs in Montreal, Canada. Participating public and private LTCFs (clusters) that provide 24-h nursing care will be assigned to either the PREPARED Trial intervention or the control (usual care) arm of the study using a covariate constrained randomization procedure. Approximately 400-600 LTC residents aged 65 and older with dementia and/or cognitive impairment will be enrolled in the study and followed for 18 weeks. Residents must be at risk of delirium, delirium-free at baseline and have resided at the facility for at least 2 weeks. Residents who are unable to communicate verbally, have a history of specific psychiatric conditions, or are receiving end-of-life care will be excluded. The PREPARED Trial intervention consists of four main components: a decision tree, an instruction manual, a training package, and a toolkit. Primary study outcomes will be assessed weekly. Functional autonomy and cognitive levels will be assessed at the beginning and end of follow-up, while information pertaining to modifiable delirium risk factors, medical consultations, and facility transfers will be collected retrospectively for the duration of the follow-up period. Primary outcomes will be reported at the level of intervention assignment. All researchers analyzing the data will be blinded to group allocation.
Discussion: This large-scale intervention study will contribute significantly to the development of evidence-based clinical guidelines for delirium prevention in this frail elderly population, as it will be the first to evaluate the efficacy of a multicomponent delirium prevention program translated into LTC clinical practice on a large scale.
Trial registration: NCT03718156 , ClinicalTrials.gov .
Keywords: Delirium; Delirium superimposed on dementia; Long-term care; Modifiable risk factors; Multicomponent intervention; Nursing.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Pilot trial of Stop Delirium! (PiTStop)--a complex intervention to prevent delirium in care homes for older people: study protocol for a cluster randomised controlled trial.Trials. 2014 Feb 5;15:47. doi: 10.1186/1745-6215-15-47. Trials. 2014. PMID: 24495514 Free PMC article. Clinical Trial.
-
Hospital Elder Life Program in Long-Term Care (HELP-LTC): A Cluster Randomized Controlled Trial.J Am Geriatr Soc. 2020 Oct;68(10):2329-2335. doi: 10.1111/jgs.16695. Epub 2020 Jul 25. J Am Geriatr Soc. 2020. PMID: 32710658 Free PMC article. Clinical Trial.
-
The trial to reduce antimicrobial use in nursing home residents with Alzheimer's disease and other dementias: study protocol for a cluster randomized controlled trial.Trials. 2019 Oct 15;20(1):594. doi: 10.1186/s13063-019-3675-y. Trials. 2019. PMID: 31615540 Free PMC article.
-
Delirium Prevention in Early Rehabilitation During Acute Hospitalization and Implementation of Programs Specifically Tailored to Older Patients with Cognitive Impairment: A Scoping Review with Meta-Analysis.J Alzheimers Dis. 2024;97(1):3-29. doi: 10.3233/JAD-230644. J Alzheimers Dis. 2024. PMID: 38073387
-
Delirium in early-stage alzheimer's disease: enhancing cognitive reserve as a possible preventive measure.J Gerontol Nurs. 2009 Mar;35(3):30-8. doi: 10.3928/00989134-20090301-06. J Gerontol Nurs. 2009. PMID: 19326827 Free PMC article. Review.
Cited by
-
The Relationship between Delirium and Dementia.Semin Neurol. 2024 Dec;44(6):732-751. doi: 10.1055/s-0044-1791543. Epub 2024 Oct 11. Semin Neurol. 2024. PMID: 39393800 Review.
-
The inter-relationship between delirium and dementia: the importance of delirium prevention.Nat Rev Neurol. 2022 Oct;18(10):579-596. doi: 10.1038/s41582-022-00698-7. Epub 2022 Aug 26. Nat Rev Neurol. 2022. PMID: 36028563 Free PMC article. Review.
References
-
- Martinez F, Martinez F, Tobar C, Tobar C, Hill N. Preventing delirium: should non-pharmacological, multicomponent interventions be used? A systematic review and meta-analysis of the literature. Age Ageing. 2015;44(2):196–204. - PubMed
-
- Lin W-L, Chen Y-F, Wang J. Factors associated with the development of delirium in elderly patients in intensive care units. J Nurs Res. 2015;23(4):322–329. - PubMed
-
- de Lange E, Verhaak P, van der Meer K. Prevalence, presentation and prognosis of delirium in older people in the population, at home and in long term care: a review. Int J Geriatr Psychiatry. 2013;28(2):127–134. - PubMed
-
- Pisani MA, Murphy TE, Van Ness PH, Araujo KL, Inouye SK. Characteristics associated with delirium in older patients in a medical intensive care unit. Arch Intern Med. 2007;167(15):13–27. - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical