A mouse model of neoadjuvant chemotherapy followed by interval cytoreductive surgery indicates impaired efficacy of perioperative cisplatin
- PMID: 34784944
- PMCID: PMC8594094
- DOI: 10.1186/s13048-021-00895-w
A mouse model of neoadjuvant chemotherapy followed by interval cytoreductive surgery indicates impaired efficacy of perioperative cisplatin
Abstract
Background: Investigate the impact of interval cytoreductive surgery (ICS) on progression in an orthotopic mouse model of ovarian cancer and the impact of chemotherapy delivered at various timelines following surgery.
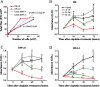
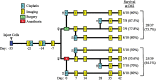
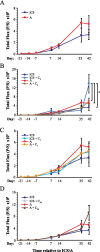
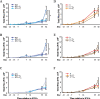
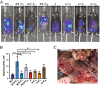
Methods: Luciferase-expressing ID8 murine ovarian cancer cells were implanted intra-bursally and IP to C57BL/7 mice. Once disease was established by bioluminescence, 2 cycles of neoadjuvant cisplatin were administered, and animals received either ICS (removal of the injected bursa/primary tumor) or anesthesia alone. Postsurgical chemotherapy was administered on the same day as the intervention (ICS/anesthesia), or on day 7 or day 28 following the intervention. Progression was quantified serially with in vivo bioluminescence imaging. Volume of ascitic fluid volume collected at necropsy was measured.
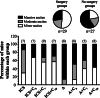
Results: Animals were matched for tumor burden at stratification. There was no accelerated growth of residual tumor after interval cytoreduction compared to controls. Animals who received chemotherapy on postoperative day (POD) 7 had better disease control compared to standard-of-care POD 28. Animals who underwent surgery had less ascites at necropsy compared to those who had anesthesia alone.
Conclusions: In this animal model, surgical wounding with suboptimal cytoreduction after neoadjuvant chemotherapy did not cause accelerated expansion of residual disease. Surgical wounding appears to impair cisplatin activity when given at time of surgery.
Keywords: Animal model; Cisplatin; Cytoreductive surgery; ID8 cells; Mouse; Neoadjuvant chemotherapy; Neoadjuvant chemotherapy treatment; Ovarian cancer; Ovarian neoplasms; Surgical oncology.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Wounding promotes ovarian cancer progression and decreases efficacy of cisplatin in a syngeneic mouse model.J Ovarian Res. 2018 Jul 4;11(1):56. doi: 10.1186/s13048-018-0428-6. J Ovarian Res. 2018. PMID: 29973223 Free PMC article.
-
Chemotherapy alone for patients 75 years and older with epithelial ovarian cancer-is interval cytoreductive surgery still needed?Am J Obstet Gynecol. 2020 Feb;222(2):170.e1-170.e11. doi: 10.1016/j.ajog.2019.07.050. Epub 2019 Aug 14. Am J Obstet Gynecol. 2020. PMID: 31421122
-
The impacts of neoadjuvant chemotherapy and of cytoreductive surgery on 10-year survival from advanced ovarian cancer.Int J Gynaecol Obstet. 2021 Jun;153(3):417-423. doi: 10.1002/ijgo.13542. Epub 2021 Jan 13. Int J Gynaecol Obstet. 2021. PMID: 33326624
-
[Combining surgery and medical treatments for ovarian cancer: Is there an optimal strategy?].Bull Cancer. 2022 Feb;109(2):197-215. doi: 10.1016/j.bulcan.2021.11.013. Epub 2022 Jan 10. Bull Cancer. 2022. PMID: 35027164 Review. French.
-
[Surgery for advanced stage ovarian cancer: Article drafted from the French Guidelines in oncology entitled "Initial management of patients with epithelial ovarian cancer" developed by FRANCOGYN, CNGOF, SFOG, GINECO-ARCAGY under the aegis of CNGOF and endorsed by INCa].Gynecol Obstet Fertil Senol. 2019 Feb;47(2):197-213. doi: 10.1016/j.gofs.2019.01.003. Epub 2019 Feb 19. Gynecol Obstet Fertil Senol. 2019. PMID: 30792175 Review. French.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. - PubMed
-
- Maringe C, Walters S, Butler J, Coleman MP, Hacker N, Hanna L, et al. Stage at diagnosis and ovarian cancer survival: evidence from the International Cancer Benchmarking Partnership. Gynecol Oncol. 2012;127:75–82. - PubMed
-
- Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

