Gasdermin D deficiency attenuates arthritis induced by traumatic injury but not autoantibody-assembled immune complexes
- PMID: 34784954
- PMCID: PMC8594229
- DOI: 10.1186/s13075-021-02668-8
Gasdermin D deficiency attenuates arthritis induced by traumatic injury but not autoantibody-assembled immune complexes
Abstract
Background: Gasdermin D (GSDMD) is cleaved by several proteases including by caspase-1, a component of intracellular protein complexes called inflammasomes. Caspase-1 also converts pro-interleukin-1β (pro-IL-1β) and pro-IL-18 into bioactive IL-1β and IL-18, respectively. GSDMD amino-terminal fragments form plasma membrane pores, which mediate the secretion of IL-1β and IL-18 and cause the inflammatory form of cell death pyroptosis. Here, we tested the hypothesis that GSDMD contributes to joint degeneration in the K/BxN serum transfer-induced arthritis (STIA) model in which autoantibodies against glucose-6-phosphate isomerase promote the formation of pathogenic immune complexes on the surface of myeloid cells, which highly express the inflammasomes. The unexpected outcomes with the STIA model prompted us to determine the role of GSDMD in the post-traumatic osteoarthritis (PTOA) model caused by meniscus ligamentous injury (MLI) based on the hypothesis that this pore-forming protein is activated by signals released from damaged joint tissues.
Methods: Gsdmd +/+ and Gsdmd-/- mice were injected with K/BxN mouse serum or subjected to MLI to cause STIA or PTOA, respectively. Paw and ankle swelling and DXA scanning were used to assess the outcomes in the STIA model whereas histopathology and micro-computed tomography (μCT) were utilized to monitor joints in the PTOA model. Murine and human joint tissues were also examined for GSDMD, IL-1β, and IL-18 expression by qPCR, immunohistochemistry, or immunoblotting.
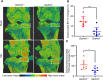
Results: GSDMD levels were higher in serum-inoculated paws compared to PBS-injected paws. Unexpectedly, ablation of GSDMD failed to reduce joint swelling and osteolysis, suggesting that GSDMD was dispensable for the pathogenesis of STIA. GSDMD levels were also higher in MLI compared to sham-operated joints. Importantly, ablation of GSDMD attenuated MLI-associated cartilage degradation (p = 0.0097), synovitis (p = 0.014), subchondral bone sclerosis (p = 0.0006), and subchondral bone plate thickness (p = 0.0174) based on histopathological and μCT analyses.
Conclusion: GSDMD plays a key role in the pathogenesis of PTOA, but not STIA, suggesting that its actions in experimental arthropathy are tissue context-specific.
Keywords: Arthritis; Bone; GSDMD; IL-1; Immune cells; Inflammasome; Inflammation; Pyroptosis.
© 2021. The Author(s).
Conflict of interest statement
Dr. Gabriel Mbalaviele is a consultant for Aclaris Therapeutics, Inc. All other authors declare that they have no competing interests.
Figures




Similar articles
-
Gasdermin-D Genetic Knockout Reduces Inflammasome-Induced Disruption of the Gut-Brain Axis After Traumatic Brain Injury.Int J Mol Sci. 2025 Apr 9;26(8):3512. doi: 10.3390/ijms26083512. Int J Mol Sci. 2025. PMID: 40331993 Free PMC article.
-
Gasdermin D and Gasdermin E Are Dispensable for Silica-Mediated IL-1β Secretion from Mouse Macrophages.Immunohorizons. 2024 Sep 1;8(9):679-687. doi: 10.4049/immunohorizons.2400019. Immunohorizons. 2024. PMID: 39264735 Free PMC article.
-
Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion.Cell Res. 2015 Dec;25(12):1285-98. doi: 10.1038/cr.2015.139. Epub 2015 Nov 27. Cell Res. 2015. PMID: 26611636 Free PMC article.
-
Uncoupled pyroptosis and IL-1β secretion downstream of inflammasome signaling.Front Immunol. 2023 Apr 6;14:1128358. doi: 10.3389/fimmu.2023.1128358. eCollection 2023. Front Immunol. 2023. PMID: 37090724 Free PMC article. Review.
-
Emerging insights into molecular mechanisms underlying pyroptosis and functions of inflammasomes in diseases.J Cell Physiol. 2020 Apr;235(4):3207-3221. doi: 10.1002/jcp.29268. Epub 2019 Oct 17. J Cell Physiol. 2020. PMID: 31621910 Review.
Cited by
-
No longer married to inflammasome signaling: the diverse interacting pathways leading to pyroptotic cell death.Biochem J. 2022 May 27;479(10):1083-1102. doi: 10.1042/BCJ20210711. Biochem J. 2022. PMID: 35608339 Free PMC article. Review.
-
Protein phosphatase PPM1A inhibition attenuates osteoarthritis via regulating TGF-β/Smad2 signaling in chondrocytes.JCI Insight. 2023 Feb 8;8(3):e166688. doi: 10.1172/jci.insight.166688. JCI Insight. 2023. PMID: 36752205 Free PMC article.
-
Mechanisms of chondrocyte cell death in osteoarthritis: implications for disease progression and treatment.J Orthop Surg Res. 2024 Sep 9;19(1):550. doi: 10.1186/s13018-024-05055-6. J Orthop Surg Res. 2024. PMID: 39252111 Free PMC article. Review.
-
The Role of Gasdermin-D-Mediated Pyroptosis in Organ Injury and Its Therapeutic Implications.Organogenesis. 2023 Dec 31;19(1):2177484. doi: 10.1080/15476278.2023.2177484. Organogenesis. 2023. PMID: 36967609 Free PMC article. Review.
-
Pyroptosis in bone loss.Apoptosis. 2023 Apr;28(3-4):293-312. doi: 10.1007/s10495-022-01807-z. Epub 2023 Jan 16. Apoptosis. 2023. PMID: 36645574 Free PMC article. Review.
References
-
- Kleyer A, Finzel S, Rech J, Manger B, Krieter M, Faustini F, et al. Bone loss before the clinical onset of rheumatoid arthritis in subjects with anticitrullinated protein antibodies. Ann Rheum Dis. 2014;73(5):854–860. - PubMed
-
- Negishi-Koga T, Gober HJ, Sumiya E, Komatsu N, Okamoto K, Sawa S, et al. Immune complexes regulate bone metabolism through FcRγ signalling. Nat Commun. 2015;6:6637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR077226/AR/NIAMS NIH HHS/United States
- R01 AR076758/AR/NIAMS NIH HHS/United States
- R21 AR077226/AR/NIAMS NIH HHS/United States
- AR075860/AR/NIAMS NIH HHS/United States
- R01 AR075860/AR/NIAMS NIH HHS/United States
- AR076758/AR/NIAMS NIH HHS/United States
- AR072623/AR/NIAMS NIH HHS/United States
- AR068972/AR/NIAMS NIH HHS/United States
- AR049192/AR/NIAMS NIH HHS/United States
- AR077616/AR/NIAMS NIH HHS/United States
- R01 AR075730/AR/NIAMS NIH HHS/United States
- AR075730/AR/NIAMS NIH HHS/United States
- AR077203/AR/NIAMS NIH HHS/United States
- AR074992/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

