Off-Target Expression of Cre-Dependent Adeno-Associated Viruses in Wild-Type C57BL/6J Mice
- PMID: 34785571
- PMCID: PMC8614227
- DOI: 10.1523/ENEURO.0363-21.2021
Off-Target Expression of Cre-Dependent Adeno-Associated Viruses in Wild-Type C57BL/6J Mice
Abstract
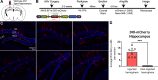
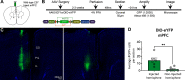
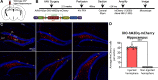
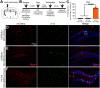
Adeno-associated viruses (AAVs) are a commonly used tool in neuroscience to efficiently label, trace, and/or manipulate neuronal populations. Highly specific targeting can be achieved through recombinase-dependent AAVs in combination with transgenic rodent lines that express Cre-recombinase in specific cell types. Visualization of viral expression is typically achieved through fluorescent reporter proteins (e.g., GFP or mCherry) packaged within the AAV genome. Although nonamplified fluorescence is usually sufficient to observe viral expression, immunohistochemical amplification of the fluorescent reporter is routinely used to improve viral visualization. In the present study, Cre-dependent AAVs were injected into the neocortex of wild-type C57BL/6J mice. While we observed weak but consistent nonamplified off-target double inverted open reading frame (DIO) expression in C57BL/6J mice, antibody amplification of the GFP or mCherry reporter revealed notable Cre-independent viral expression. Off-target expression of DIO constructs in wild-type C57BL/6J mice occurred independent of vendor, AAV serotype, or promoter. We also evaluated whether Cre-independent expression had functional effects via designer receptors exclusively activated by designer drugs (DREADDs). The DREADD agonist C21 (compound 21) had no effect on contextual fear conditioning or c-Fos expression in DIO-hM3Dq-mCherry+ cells of C57BL/6J mice. Together, our results indicate that DIO constructs have off-target expression in wild-type subjects. Our findings are particularly important for the design of experiments featuring sensitive systems and/or quantitative measurements that could be negatively impacted by off-target expression.
Keywords: Cre/loxP; DREADDs; Immunofluorescence; antibody amplification; c-Fos; double inverted open reading frame; fear conditioning.
Copyright © 2021 Botterill et al.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
