Interleukin-6 Receptor Blockade in Treatment-Refractory MOG-IgG-Associated Disease and Neuromyelitis Optica Spectrum Disorders
- PMID: 34785575
- PMCID: PMC8596357
- DOI: 10.1212/NXI.0000000000001100
Interleukin-6 Receptor Blockade in Treatment-Refractory MOG-IgG-Associated Disease and Neuromyelitis Optica Spectrum Disorders
Abstract
Background and objectives: To evaluate the long-term safety and efficacy of tocilizumab (TCZ), a humanized anti-interleukin-6 receptor antibody in myelin oligodendrocyte glycoprotein-IgG-associated disease (MOGAD) and neuromyelitis optica spectrum disorders (NMOSD).
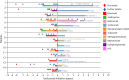
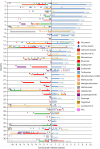
Methods: Annualized relapse rate (ARR), Expanded Disability Status Scale score, MRI, autoantibody titers, pain, and adverse events were retrospectively evaluated in 57 patients with MOGAD (n = 14), aquaporin-4 (AQP4)-IgG seropositive (n = 36), and seronegative NMOSD (n = 7; 12%), switched to TCZ from previous immunotherapies, particularly rituximab.
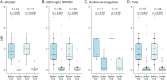
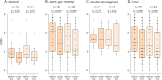
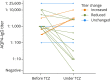
Results: Patients received TCZ for 23.8 months (median; interquartile range 13.0-51.1 months), with an IV dose of 8.0 mg/kg (median; range 6-12 mg/kg) every 31.6 days (mean; range 26-44 days). For MOGAD, the median ARR decreased from 1.75 (range 0.5-5) to 0 (range 0-0.9; p = 0.0011) under TCZ. A similar effect was seen for AQP4-IgG+ (ARR reduction from 1.5 [range 0-5] to 0 [range 0-4.2]; p < 0.001) and for seronegative NMOSD (from 3.0 [range 1.0-3.0] to 0.2 [range 0-2.0]; p = 0.031). During TCZ, 60% of all patients were relapse free (79% for MOGAD, 56% for AQP4-IgG+, and 43% for seronegative NMOSD). Disability follow-up indicated stabilization. MRI inflammatory activity decreased in MOGAD (p = 0.04; for the brain) and in AQP4-IgG+ NMOSD (p < 0.001; for the spinal cord). Chronic pain was unchanged. Regarding only patients treated with TCZ for at least 12 months (n = 44), ARR reductions were confirmed, including the subgroups of MOGAD (n = 11) and AQP4-IgG+ patients (n = 28). Similarly, in the group of patients treated with TCZ for at least 12 months, 59% of them were relapse free, with 73% for MOGAD, 57% for AQP4-IgG+, and 40% for patients with seronegative NMOSD. No severe or unexpected safety signals were observed. Add-on therapy showed no advantage compared with TCZ monotherapy.
Discussion: This study provides Class III evidence that long-term TCZ therapy is safe and reduces relapse probability in MOGAD and AQP4-IgG+ NMOSD.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
