Decidual NK cells kill Zika virus-infected trophoblasts
- PMID: 34785597
- PMCID: PMC8617421
- DOI: 10.1073/pnas.2115410118
Decidual NK cells kill Zika virus-infected trophoblasts
Abstract
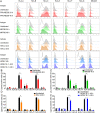
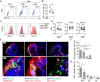
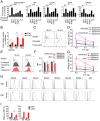
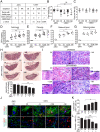
Zika virus (ZIKV) during pregnancy infects fetal trophoblasts and causes placental damage and birth defects including microcephaly. Little is known about the anti-ZIKV cellular immune response at the maternal-fetal interface. Decidual natural killer cells (dNK), which directly contact fetal trophoblasts, are the dominant maternal immune cells in the first-trimester placenta, when ZIKV infection is most hazardous. Although dNK express all the cytolytic molecules needed to kill, they usually do not kill infected fetal cells but promote placentation. Here, we show that dNK degranulate and kill ZIKV-infected placental trophoblasts. ZIKV infection of trophoblasts causes endoplasmic reticulum (ER) stress, which makes them dNK targets by down-regulating HLA-C/G, natural killer (NK) inhibitory receptor ligands that help maintain tolerance of the semiallogeneic fetus. ER stress also activates the NK activating receptor NKp46. ZIKV infection of Ifnar1 -/- pregnant mice results in high viral titers and severe intrauterine growth restriction, which are exacerbated by depletion of NK or CD8 T cells, indicating that killer lymphocytes, on balance, protect the fetus from ZIKV by eliminating infected cells and reducing the spread of infection.
Keywords: ER stress; ZIKV; decidual NK; extravillous trophoblast; pregnancy.
Conflict of interest statement
The authors declare no competing interest.
Figures

Similar articles
-
Expression of KIR2DS1 by decidual natural killer cells increases their ability to control placental HCMV infection.Proc Natl Acad Sci U S A. 2016 Dec 27;113(52):15072-15077. doi: 10.1073/pnas.1617927114. Epub 2016 Dec 12. Proc Natl Acad Sci U S A. 2016. PMID: 27956621 Free PMC article.
-
Zika virus infection of first trimester trophoblast cells affects cell migration, metabolism and immune homeostasis control.J Cell Physiol. 2021 Jul;236(7):4913-4925. doi: 10.1002/jcp.30203. Epub 2020 Dec 10. J Cell Physiol. 2021. PMID: 33305387
-
HSV-2 enhances ZIKV infection of the placenta and induces apoptosis in first-trimester trophoblast cells.Am J Reprod Immunol. 2016 Nov;76(5):348-357. doi: 10.1111/aji.12578. Epub 2016 Sep 10. Am J Reprod Immunol. 2016. PMID: 27613665
-
Cytotoxic potential of decidual NK cells and CD8+ T cells awakened by infections.J Reprod Immunol. 2017 Feb;119:85-90. doi: 10.1016/j.jri.2016.08.001. Epub 2016 Aug 2. J Reprod Immunol. 2017. PMID: 27523927 Free PMC article. Review.
-
Viral-Immune Cell Interactions at the Maternal-Fetal Interface in Human Pregnancy.Front Immunol. 2020 Oct 7;11:522047. doi: 10.3389/fimmu.2020.522047. eCollection 2020. Front Immunol. 2020. PMID: 33117336 Free PMC article. Review.
Cited by
-
The mechanisms underlying the immune control of Zika virus infection at the maternal-fetal interface.Front Immunol. 2022 Nov 22;13:1000861. doi: 10.3389/fimmu.2022.1000861. eCollection 2022. Front Immunol. 2022. PMID: 36483552 Free PMC article.
-
Local immune recognition of trophoblast in early human pregnancy: controversies and questions.Nat Rev Immunol. 2023 Apr;23(4):222-235. doi: 10.1038/s41577-022-00777-2. Epub 2022 Oct 3. Nat Rev Immunol. 2023. PMID: 36192648 Free PMC article. Review.
-
Costimulatory CD226 Signaling Regulates Proliferation of Memory-like NK Cells in Healthy Individuals with Latent Mycobacterium tuberculosis Infection.Int J Mol Sci. 2022 Oct 25;23(21):12838. doi: 10.3390/ijms232112838. Int J Mol Sci. 2022. PMID: 36361628 Free PMC article.
-
Apoptosis during ZIKA Virus Infection: Too Soon or Too Late?Int J Mol Sci. 2022 Jan 24;23(3):1287. doi: 10.3390/ijms23031287. Int J Mol Sci. 2022. PMID: 35163212 Free PMC article. Review.
-
The Innate Defense in the Zika-Infected Placenta.Pathogens. 2022 Nov 24;11(12):1410. doi: 10.3390/pathogens11121410. Pathogens. 2022. PMID: 36558744 Free PMC article. Review.
References
-
- Apps R., Gardner L., Moffett A., A critical look at HLA-G. Trends Immunol. 29, 313–321 (2008). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

