Efficient Delivery of Antibodies Intracellularly by Co-Assembly with Hexahistidine-Metal Assemblies (HmA)
- PMID: 34785893
- PMCID: PMC8579864
- DOI: 10.2147/IJN.S332279
Efficient Delivery of Antibodies Intracellularly by Co-Assembly with Hexahistidine-Metal Assemblies (HmA)
Abstract
Purpose: There has been a substantial global market for antibodies, which are based on extracellular targets. Binding intracellular targets by antibodies will bring new chances in antibody therapeutics and a huge market increase. We aim to evaluate the efficiency of a novel delivery system of His6-metal assembly (HmA) in delivering intracellular antibodies and biofunctions of delivered antibodies.
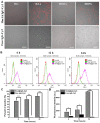
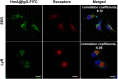
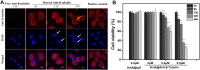
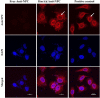
Methods: In this study, the physicochemical properties of HmA@Antibodies generated through co-assembling with antibodies and HmA were well characterized by dynamic light scatter. The cytotoxicity of HmA@Antibodies was investigated by Cell Counting Kit-8 (CCK-8). The endocytic kinetics and lysosome escape process of HmA@Antibodies were studied by flow cytometry and fluorescent staining imaging, respectively. Compared to the commercialized positive control, the intracellular delivery efficiency by HmA@Antibodies and biofunctions of delivered antibodies were evaluated by fluorescent imaging and CCK-8.
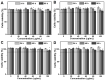
Results: Various antibodies (IgG, anti-β-tubulin and anti-NPC) could co-assemble with HmA under a gentle condition, producing nano-sized (~150 nm) and positively charged (~+30 eV) HmA@Antibodies particles with narrow size distribution (PDI ~ 0.15). HmA displayed very low cytotoxicity to divers cells (DCs, HeLa, HCECs, and HRPE) even after 96 h for the feeding concentration ≤100 μg mL-1, and fast escape from endosomes. In the case of delivery IgG, the delivery efficiency into alive cells of HmA was better than a commercial protein delivery reagent (PULSin). For cases of the anti-β-tubulin and anti-NPC, HmA showed comparable delivery efficiency to their positive controls, but HmA with ability to deliver these antibodies into alive cells was still superior to positive controls delivering antibodies into dead cells through punching holes.
Conclusion: Our results indicate that this strategy is a feasible way to deliver various antibodies intracellularly while preserving their functions, which has great potential in various applications and treating many refractory diseases by intracellular antibody delivery.
Keywords: antibody; coordination polymer; intracellular delivery; nanocarrier; peptide assembly.
© 2021 Wei et al.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures


Similar articles
-
Efficient delivery of cytosolic proteins by protein-hexahistidine-metal co-assemblies.Acta Biomater. 2021 Jul 15;129:199-208. doi: 10.1016/j.actbio.2021.05.001. Epub 2021 May 12. Acta Biomater. 2021. PMID: 33991683
-
Hexahistidine-metal assemblies: A promising drug delivery system.Acta Biomater. 2019 May;90:441-452. doi: 10.1016/j.actbio.2019.03.058. Epub 2019 Apr 3. Acta Biomater. 2019. PMID: 30953803
-
RNA-binding peptide and endosomal escape-assisting peptide (L2) improved siRNA delivery by the hexahistidine-metal assembly.J Mater Chem B. 2024 Oct 17;12(40):10309-10319. doi: 10.1039/d4tb01433b. J Mater Chem B. 2024. PMID: 39282740
-
Functional antibody delivery: Advances in cellular manipulation.Adv Drug Deliv Rev. 2023 Jan;192:114586. doi: 10.1016/j.addr.2022.114586. Epub 2022 Oct 22. Adv Drug Deliv Rev. 2023. PMID: 36280179 Review.
-
Antibody Delivery for Intracellular Targets: Emergent Therapeutic Potential.Bioconjug Chem. 2019 Apr 17;30(4):1028-1041. doi: 10.1021/acs.bioconjchem.9b00025. Epub 2019 Mar 18. Bioconjug Chem. 2019. PMID: 30830750 Free PMC article. Review.
Cited by
-
Short Peptides as Powerful Arsenal for Smart Fighting Cancer.Cancers (Basel). 2024 Sep 24;16(19):3254. doi: 10.3390/cancers16193254. Cancers (Basel). 2024. PMID: 39409876 Free PMC article. Review.
-
Peptide-drug co-assembling: A potent armament against cancer.Theranostics. 2023 Sep 25;13(15):5322-5347. doi: 10.7150/thno.87356. eCollection 2023. Theranostics. 2023. PMID: 37908727 Free PMC article. Review.
-
Emulsifying Lipiodol with pH-sensitive DOX@HmA nanoparticles for hepatocellular carcinoma TACE treatment eliminate metastasis.Mater Today Bio. 2023 Nov 29;23:100873. doi: 10.1016/j.mtbio.2023.100873. eCollection 2023 Dec. Mater Today Bio. 2023. PMID: 38149018 Free PMC article.
-
Novel anti-pyroptosis drug loaded on metal-organic framework for intervertebral disc degeneration therapy.Mater Today Bio. 2025 Apr 5;32:101729. doi: 10.1016/j.mtbio.2025.101729. eCollection 2025 Jun. Mater Today Bio. 2025. PMID: 40275959 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

