Decellularized extracellular matrix loaded with IPFP-SC for repairing rabbit osteochondral defects
- PMID: 34786041
- PMCID: PMC8581932
Decellularized extracellular matrix loaded with IPFP-SC for repairing rabbit osteochondral defects
Abstract
Background: Tissue engineering is widely applied to treat osteochondral damage in osteoarthritis (OA). However, the superposition of seed cells, material scaffolds, inducing factors, and microenvironmental factors limit their practical application. We intended to develop a novel tissue engineering method for improving the repairment of osteochondral damage and to discuss its effect on repairing osteochondral defects.
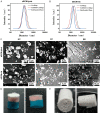
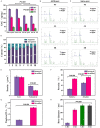
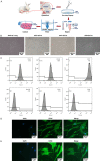
Methods: The combined decellularization methods of physics, chemistry and enzymes were used to decellularize rabbit rib cartilage and articular cartilage, and rabbit decellularizated osteochondral composite scaffolds were prepared. The structure and organization of the scaffolds were analyzed. We extracted and identified infrapatellar fat pad stem cells (IPFP-SCs) from healthy rabbits and OA rabbit, which were different in viability, migration, osteogenic and chondrogenic differentiation. Finally, a variety of decellularizated bone cartilage composite scaffolds were loaded with rabbit IPFP-SC for in vitro and in vivo studies.
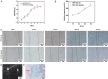
Results: The decellularization effect was strong, and the organic ingredients were lost. The layered scaffold showed lower density, greater porosity, larger pore size and water absorption than the whole scaffold, but the mechanical properties of the two scaffolds were low. IPFP-SCs were successfully extracted, and the migration and cartilage ability of IPFP-SCs in OA group were weak. The decellularized scaffold showed a high biocompatibility. The structure and composition of osteochondral promoted osteogenic differentiation and chondrogenic differentiation of IPFP-SCs. Moreover, the decellularized extracellular matrix loaded with IPFP-SC had the strongest repairing effect.
Conclusion: The decellularized extracellular matrix loaded with IPFP-SC showed a better repair effect on rabbit osteochondral defects.
Keywords: Tissue engineering; decellularized extracellular matrix; infrapatellar fat pad stem cells; osteoarthritis; osteochondral defects.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures







Similar articles
-
Culture of Mesenchymal Stem Cells Derived From the Infrapatellar Fat Pad Without Enzyme and Preliminary Study on the Repair of Articular Cartilage Defects in Rabbits.Front Bioeng Biotechnol. 2022 Aug 17;10:889306. doi: 10.3389/fbioe.2022.889306. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36061444 Free PMC article.
-
Extracellular matrix derived from allogenic decellularized bone marrow mesenchymal stem cell sheets for the reconstruction of osteochondral defects in rabbits.Acta Biomater. 2020 Dec;118:54-68. doi: 10.1016/j.actbio.2020.10.022. Epub 2020 Oct 15. Acta Biomater. 2020. PMID: 33068746
-
Scaffold-Free Cartilage Construct from Infrapatellar Fat Pad Stem Cells for Cartilage Restoration.Tissue Eng Part A. 2022 Mar;28(5-6):199-211. doi: 10.1089/ten.TEA.2020.0167. Epub 2020 Oct 23. Tissue Eng Part A. 2022. PMID: 32972295
-
Osteochondral Tissue Engineering Dilemma: Scaffolding Trends in Regenerative Medicine.Stem Cell Rev Rep. 2023 Aug;19(6):1615-1634. doi: 10.1007/s12015-023-10545-x. Epub 2023 Apr 19. Stem Cell Rev Rep. 2023. PMID: 37074547 Review.
-
Recent Advance in Source, Property, Differentiation, and Applications of Infrapatellar Fat Pad Adipose-Derived Stem Cells.Stem Cells Int. 2020 Mar 7;2020:2560174. doi: 10.1155/2020/2560174. eCollection 2020. Stem Cells Int. 2020. PMID: 32215015 Free PMC article. Review.
Cited by
-
Decellularized laser micro-patterned osteochondral implants exhibit zonal recellularization and self-fixing for osteochondral regeneration in a goat model.J Orthop Translat. 2024 May 10;46:18-32. doi: 10.1016/j.jot.2024.04.005. eCollection 2024 May. J Orthop Translat. 2024. PMID: 38774916 Free PMC article.
-
Knee adipose tissue: from its implication in osteoarthritis to its supposed role in tissue engineering.NPJ Aging. 2025 Feb 3;11(1):5. doi: 10.1038/s41514-025-00195-3. NPJ Aging. 2025. PMID: 39900591 Free PMC article.
-
Evaluation of Chondral Defect Repair Using Human Fibronectin Adhesion Assay-Derived Chondroprogenitors Suspended in Lyophilized Fetal Collagen Scaffold: An Ex Vivo Osteochondral Unit Model Study.Indian J Orthop. 2024 May 31;58(8):991-1000. doi: 10.1007/s43465-024-01192-6. eCollection 2024 Aug. Indian J Orthop. 2024. PMID: 39087036 Free PMC article.
References
-
- Goldring SR, Goldring MB. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat Rev Rheumatol. 2016;12:632–644. - PubMed
-
- Mapp PI, Walsh DA. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–398. - PubMed
LinkOut - more resources
Full Text Sources
