Fungal dysbiosis of the gut microbiota is associated with colorectal cancer in Chinese patients
- PMID: 34786058
- PMCID: PMC8581944
Fungal dysbiosis of the gut microbiota is associated with colorectal cancer in Chinese patients
Abstract
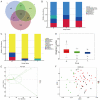
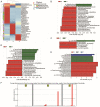
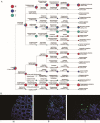
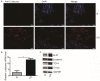
Changes in bacteria and virions are associated with colorectal cancer (CRC). However, the fungal microbiota in the intestines of CRC patients remains largely unexamined. We identified differences in the intestinal fungal microbiota between healthy persons and patients with colorectal polyps or CRC. Using second-generation sequencing technology, we sequenced and aligned the ITS1 regions of fungi collected from fecal samples. We found a significant increase in the Candida albicans levels in the guts of CRC patients. Dectin-1 is a C-type lectin receptor that recognizes β-1,3-glucan in the cell walls of most fungi and is expressed by many cell types, including dendritic cells, macrophages, and monocytes. However, the mechanisms controlling the expressions and functions of dectin-1 in intestinal epithelial cells (IECs) remain unclear. Furthermore, the putative effects of C. albicans on IECs are unknown. C. albicans induces the proliferation of IECs by activating the Wnt signaling pathway, and the Wnt pathway contributes to the development of CRC. Mice infected with C. albicans show an activation of the Wnt pathway. Therefore, IECs may recognize the activation of the Wnt pathway by C. albicans through dectin-1 to promote the development of CRC.
Keywords: Candida albicans; Fungi; Wnt; intestinal epithelial cells; proliferation; sequencing.
AJTR Copyright © 2021.
Conflict of interest statement
None.
Figures


Similar articles
-
Human intestinal epithelial cells respond to β-glucans via Dectin-1 and Syk.Eur J Immunol. 2014 Dec;44(12):3729-40. doi: 10.1002/eji.201444876. Epub 2014 Oct 27. Eur J Immunol. 2014. PMID: 25251945 Clinical Trial.
-
Gut microbiota from colorectal cancer patients enhances the progression of intestinal adenoma in Apcmin/+ mice.EBioMedicine. 2019 Oct;48:301-315. doi: 10.1016/j.ebiom.2019.09.021. Epub 2019 Oct 5. EBioMedicine. 2019. PMID: 31594750 Free PMC article.
-
Human intestinal epithelial cells can internalize luminal fungi via LC3-associated phagocytosis.Front Immunol. 2023 Mar 8;14:1142492. doi: 10.3389/fimmu.2023.1142492. eCollection 2023. Front Immunol. 2023. PMID: 36969163 Free PMC article.
-
Gut Microbiota Dysbiosis Drives the Development of Colorectal Cancer.Digestion. 2021;102(4):508-515. doi: 10.1159/000508328. Epub 2020 Sep 15. Digestion. 2021. PMID: 32932258 Review.
-
Fungi in Gastrointestinal Tracts of Human and Mice: from Community to Functions.Microb Ecol. 2018 May;75(4):821-829. doi: 10.1007/s00248-017-1105-9. Epub 2017 Nov 6. Microb Ecol. 2018. PMID: 29110065 Review.
Cited by
-
The mycobiome as integral part of the gut microbiome: crucial role of symbiotic fungi in health and disease.Gut Microbes. 2024 Jan-Dec;16(1):2440111. doi: 10.1080/19490976.2024.2440111. Epub 2024 Dec 15. Gut Microbes. 2024. PMID: 39676474 Free PMC article. Review.
-
Mycobiome: an underexplored kingdom in cancer.Microbiol Mol Biol Rev. 2025 Jun 25;89(2):e0026124. doi: 10.1128/mmbr.00261-24. Epub 2025 Mar 14. Microbiol Mol Biol Rev. 2025. PMID: 40084887 Review.
-
The role of intestinal stem cell within gut homeostasis: Focusing on its interplay with gut microbiota and the regulating pathways.Int J Biol Sci. 2022 Aug 8;18(13):5185-5206. doi: 10.7150/ijbs.72600. eCollection 2022. Int J Biol Sci. 2022. PMID: 35982910 Free PMC article. Review.
-
The Role of C-Type Lectin Receptor Signaling in the Intestinal Microbiota-Inflammation-Cancer Axis.Front Immunol. 2022 May 10;13:894445. doi: 10.3389/fimmu.2022.894445. eCollection 2022. Front Immunol. 2022. PMID: 35619716 Free PMC article. Review.
-
Tumor-related fungi and crosstalk with gut fungi in the tumor microenvironment.Cancer Biol Med. 2024 Nov 27;21(11):977-94. doi: 10.20892/j.issn.2095-3941.2024.0240. Cancer Biol Med. 2024. PMID: 39601429 Free PMC article. Review.
References
-
- Konstantinov SR, Kuipers EJ, Peppelenbosch MP. Functional genomic analyses of the gut microbiota for CRC screening. Nat Rev Gastroenterol Hepatol. 2013;10:741–745. - PubMed
LinkOut - more resources
Full Text Sources
