On the Selectivity of Heparan Sulfate Recognition by SARS-CoV-2 Spike Glycoprotein
- PMID: 34786180
- PMCID: PMC8525342
- DOI: 10.1021/acsmedchemlett.1c00343
On the Selectivity of Heparan Sulfate Recognition by SARS-CoV-2 Spike Glycoprotein
Abstract
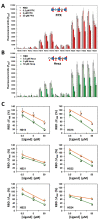
SARS-CoV-2 infects human cells through its surface spike glycoprotein (SgP), which relies on host cell surface heparan sulfate (HS) proteoglycans that facilitate interaction with the ACE2 receptor. Targeting this process could lead to inhibitors of early steps in viral entry. Screening a microarray of 24 HS oligosaccharides against recombinant S1 and receptor-binding domain (RBD) proteins led to identification of only eight sequences as potent antagonists; results that were supported by detailed dual-filter computational studies. Competitive studies using the HS microarray suggested almost equivalent importance of IdoA2S-GlcNS6S and GlcNS3S structures, which were supported by affinity studies. Exhaustive virtual screening on a library of >93 000 sequences led to a novel pharmacophore with at least two 3-O-sulfated GlcN residues that can engineer unique selectivity in recognizing the RBD. This work puts forward the key structural motif in HS that should lead to potent and selective HS or HS-like agents against SARS-CoV-2.
© 2021 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Heparan Sulfate Proteoglycans as Attachment Factor for SARS-CoV-2.ACS Cent Sci. 2021 Jun 23;7(6):1009-1018. doi: 10.1021/acscentsci.1c00010. Epub 2021 Jun 2. ACS Cent Sci. 2021. PMID: 34235261 Free PMC article.
-
Interactions between heparin and SARS-CoV-2 spike glycoprotein RBD from omicron and other variants.Front Mol Biosci. 2022 Aug 15;9:912887. doi: 10.3389/fmolb.2022.912887. eCollection 2022. Front Mol Biosci. 2022. PMID: 36046608 Free PMC article.
-
Heparan sulfate proteoglycans as attachment factor for SARS-CoV-2.bioRxiv [Preprint]. 2021 Jan 4:2020.05.10.087288. doi: 10.1101/2020.05.10.087288. bioRxiv. 2021. Update in: ACS Cent Sci. 2021 Jun 23;7(6):1009-1018. doi: 10.1021/acscentsci.1c00010. PMID: 32511404 Free PMC article. Updated. Preprint.
-
The diverse role of heparan sulfate and other GAGs in SARS-CoV-2 infections and therapeutics.Carbohydr Polym. 2023 Jan 1;299:120167. doi: 10.1016/j.carbpol.2022.120167. Epub 2022 Sep 28. Carbohydr Polym. 2023. PMID: 36876764 Free PMC article. Review.
-
Inhibition of S-protein RBD and hACE2 Interaction for Control of SARSCoV- 2 Infection (COVID-19).Mini Rev Med Chem. 2021;21(6):689-703. doi: 10.2174/1389557520666201117111259. Mini Rev Med Chem. 2021. PMID: 33208074 Review.
Cited by
-
Assessing Genetic Algorithm-Based Docking Protocols for Prediction of Heparin Oligosaccharide Binding Geometries onto Proteins.Biomolecules. 2023 Nov 9;13(11):1633. doi: 10.3390/biom13111633. Biomolecules. 2023. PMID: 38002315 Free PMC article.
-
Glycosaminoglycans: What Remains To Be Deciphered?JACS Au. 2023 Mar 2;3(3):628-656. doi: 10.1021/jacsau.2c00569. eCollection 2023 Mar 27. JACS Au. 2023. PMID: 37006755 Free PMC article. Review.
-
Phagocyte-expressed glycosaminoglycans promote capture of alphaviruses from the blood circulation in a host species-specific manner.PNAS Nexus. 2024 Mar 20;3(4):pgae119. doi: 10.1093/pnasnexus/pgae119. eCollection 2024 Apr. PNAS Nexus. 2024. PMID: 38560529 Free PMC article.
-
Controlling the Sulfation Density of Glycosaminoglycan Glycopolymer Mimetics Enables High Antiviral Activity against SARS-CoV-2 and Reduces Anticoagulant Activity.Biomacromolecules. 2025 Aug 11;26(8):5169-5181. doi: 10.1021/acs.biomac.5c00576. Epub 2025 Jul 6. Biomacromolecules. 2025. PMID: 40619669 Free PMC article.
-
HS, an Ancient Molecular Recognition and Information Storage Glycosaminoglycan, Equips HS-Proteoglycans with Diverse Matrix and Cell-Interactive Properties Operative in Tissue Development and Tissue Function in Health and Disease.Int J Mol Sci. 2023 Jan 6;24(2):1148. doi: 10.3390/ijms24021148. Int J Mol Sci. 2023. PMID: 36674659 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

