Modulation of pulsatile GnRH dynamics across the ovarian cycle via changes in the network excitability and basal activity of the arcuate kisspeptin network
- PMID: 34787076
- PMCID: PMC8651288
- DOI: 10.7554/eLife.71252
Modulation of pulsatile GnRH dynamics across the ovarian cycle via changes in the network excitability and basal activity of the arcuate kisspeptin network
Abstract
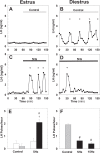
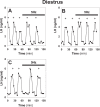
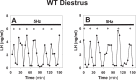
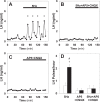
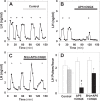
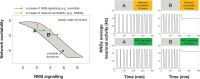
Pulsatile GnRH release is essential for normal reproductive function. Kisspeptin secreting neurons found in the arcuate nucleus, known as KNDy neurons for co-expressing neurokinin B, and dynorphin, drive pulsatile GnRH release. Furthermore, gonadal steroids regulate GnRH pulsatile dynamics across the ovarian cycle by altering KNDy neurons' signalling properties. However, the precise mechanism of regulation remains mostly unknown. To better understand these mechanisms, we start by perturbing the KNDy system at different stages of the estrous cycle using optogenetics. We find that optogenetic stimulation of KNDy neurons stimulates pulsatile GnRH/LH secretion in estrous mice but inhibits it in diestrous mice. These in vivo results in combination with mathematical modelling suggest that the transition between estrus and diestrus is underpinned by well-orchestrated changes in neuropeptide signalling and in the excitability of the KNDy population controlled via glutamate signalling. Guided by model predictions, we show that blocking glutamate signalling in diestrous animals inhibits LH pulses, and that optic stimulation of the KNDy population mitigates this inhibition. In estrous mice, disruption of glutamate signalling inhibits pulses generated via sustained low-frequency optic stimulation of the KNDy population, supporting the idea that the level of network excitability is critical for pulse generation. Our results reconcile previous puzzling findings regarding the estradiol-dependent effect that several neuromodulators have on the GnRH pulse generator dynamics. Therefore, we anticipate our model to be a cornerstone for a more quantitative understanding of the pathways via which gonadal steroids regulate GnRH pulse generator dynamics. Finally, our results could inform useful repurposing of drugs targeting the glutamate system in reproductive therapy.
Keywords: GnRH pulse generator; KNDy; mathematical model; mouse; neuroscience; optogenetics.
© 2021, Voliotis et al.
Conflict of interest statement
MV, XL, RD, GL, DI, CM, KO, KT No competing interests declared
Figures




Similar articles
-
Suppression of the GnRH pulse generator by neurokinin B involves a κ-opioid receptor-dependent mechanism.Endocrinology. 2012 Oct;153(10):4894-904. doi: 10.1210/en.2012-1574. Epub 2012 Aug 17. Endocrinology. 2012. PMID: 22903614
-
Deletion of Nuclear Progesterone Receptors From Kisspeptin Cells Does Not Impair Negative Feedback in Female Mice.Endocrinology. 2024 Aug 27;165(10):bqae121. doi: 10.1210/endocr/bqae121. Endocrinology. 2024. PMID: 39253941
-
Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes.Endocrinology. 2013 Nov;154(11):4259-69. doi: 10.1210/en.2013-1331. Epub 2013 Aug 19. Endocrinology. 2013. PMID: 23959940 Free PMC article.
-
Cellular and molecular mechanisms regulating the KNDy neuronal activities to generate and modulate GnRH pulse in mammals.Front Neuroendocrinol. 2022 Jan;64:100968. doi: 10.1016/j.yfrne.2021.100968. Epub 2021 Nov 19. Front Neuroendocrinol. 2022. PMID: 34808231 Review.
-
Regulation of GnRH pulsatility in ewes.Reproduction. 2018 Sep;156(3):R83-R99. doi: 10.1530/REP-18-0127. Epub 2018 Jun 7. Reproduction. 2018. PMID: 29880718 Free PMC article. Review.
Cited by
-
A New Hope for Woman with Vasomotor Symptoms: Neurokinin B Antagonists.J Clin Med. 2025 Feb 21;14(5):1438. doi: 10.3390/jcm14051438. J Clin Med. 2025. PMID: 40094924 Free PMC article. Review.
-
Impacts of sex differences on optogenetic, chemogenetic, and calcium-imaging tools.Curr Opin Neurobiol. 2024 Feb;84:102817. doi: 10.1016/j.conb.2023.102817. Epub 2023 Dec 1. Curr Opin Neurobiol. 2024. PMID: 38042130 Free PMC article. Review.
-
Neuronal network dynamics in the posterodorsal amygdala: shaping reproductive hormone pulsatility.J R Soc Interface. 2024 Aug;21(217):20240143. doi: 10.1098/rsif.2024.0143. Epub 2024 Aug 28. J R Soc Interface. 2024. PMID: 39193642 Free PMC article.
-
Glutamatergic Input From Arcuate Nucleus Kiss1 Neurons to Preoptic Kiss1 Neurons Is Required for LH Surge in Female Mice.Endocrinology. 2025 Jan 6;166(2):bqaf015. doi: 10.1210/endocr/bqaf015. Endocrinology. 2025. PMID: 39865886
-
Kisspeptin-neuron control of LH pulsatility and ovulation.Front Endocrinol (Lausanne). 2022 Nov 21;13:951938. doi: 10.3389/fendo.2022.951938. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36479214 Free PMC article. Review.
References
-
- Arias P, Jarry H, Leonhardt S, Moguilevsky JA, Wuttke W. Estradiol modulates the LH release response to N-methyl-D-aspartate in adult female rats: studies on hypothalamic luteinizing hormone-releasing hormone and neurotransmitter release. Neuroendocrinology. 1993;57:710–715. doi: 10.1159/000126429. - DOI - PubMed
-
- Bonavera JJ, Sahu A, Kalra SP, Kalra PS. The hypothalamic peptides, beta-endorphin, neuropeptide K and interleukin-1 beta, and the opiate morphine, enhance the excitatory amino acid-induced LH release under the influence of gonadal steroids. Journal of Neuroendocrinology. 1994;6:557–564. doi: 10.1111/j.1365-2826.1994.tb00619.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

