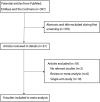
Bone Marrow-Derived Stem Cells for Patients with Liver Cirrhosis: A Systematic Review and Meta-analysis
- PMID: 34787095
- PMCID: PMC8975511
- DOI: 10.5152/tjg.2021.19694
Bone Marrow-Derived Stem Cells for Patients with Liver Cirrhosis: A Systematic Review and Meta-analysis
Abstract
Background: To date, studies have shown inconsistent results of treatment with bone marrow-derived stem cells (BMDSC) for patients with liver cirrhosis. This study aims to compare the efficacy and safety of BMDSC and standard therapy for liver cirrhosis.
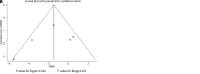
Methods: Articles from PubMed, Embase, and the Cochrane library were searched from inception to April 2018. The index included Model for End-stage Liver Disease (MELD), alanine aminotransferase (ALT), albumin, total bilirubin (TBIL), prothrombin time (PT), Child-Pugh score, and all-cause mortality.
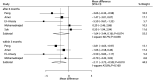
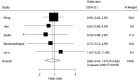
Results: A total of 9 studies with a total of 424 patients with liver cirrhosis were included in final meta-analysis. BMDSC therapy was associated with lower MELD within 3 months (P = .010), while it had no significant impact on MELD after 6 months (P = .074). There were no differences between BMDSC and standard therapy for ALT within 3 months (P = .336) and after 6 months (P = .379). BMDSC did not affect albumin level within 3 months (P = .196) and after 6 months (P = .840). BMDSC reduced the TBIL level within 3 months (P = .037) and was not associated with the TBIL level after 6 months (P = .914). There were no differences between BMDSC and standard therapy for PT within 3 months (P = .167) and after 6 months (P = .484). The Child-Pugh scores within 3 months (P = .342) and after 6 months (P = .133) were not associated with BMDSC treatment for liver cirrhosis patients. Finally, the BMDSC was not associated with the risk of all-cause mortality, as compared with standard therapy (P = .622).
Conclusions: BMDSC treatment for patients with liver cirrhosis could improve short-term MELD and TBIL, but not the risk of mortality, as compared with standard therapy.
Conflict of interest statement
Figures





Similar articles
-
Effectiveness and Safety of Stem Cell Therapy in Liver Cirrhosis: A Meta-Analysis of Randomized Controlled Trials.Altern Ther Health Med. 2024 Jun;30(6):246-253. Altern Ther Health Med. 2024. PMID: 37944965
-
Enhanced therapeutic effects of umbilical cord mesenchymal stem cells after prolonged treatment for HBV-related liver failure and liver cirrhosis.Stem Cell Res Ther. 2020 Jul 10;11(1):277. doi: 10.1186/s13287-020-01787-4. Stem Cell Res Ther. 2020. PMID: 32650827 Free PMC article.
-
Meta-analysis on last ten years of clinical injection of bone marrow-derived and umbilical cord MSC to reverse cirrhosis or rescue patients with acute-on-chronic liver failure.Stem Cell Res Ther. 2023 Sep 23;14(1):267. doi: 10.1186/s13287-023-03494-2. Stem Cell Res Ther. 2023. PMID: 37742014 Free PMC article.
-
Bone marrow-derived mesenchymal stem cell therapy for decompensated liver cirrhosis: a meta-analysis.World J Gastroenterol. 2014 Oct 14;20(38):14051-7. doi: 10.3748/wjg.v20.i38.14051. World J Gastroenterol. 2014. PMID: 25320545 Free PMC article. Review.
-
Autologous bone marrow stem cell transplantation via the hepatic artery for the treatment of hepatitis B virus-related cirrhosis: a PRISMA-compliant meta-analysis based on the Chinese population.Stem Cell Res Ther. 2020 Mar 5;11(1):104. doi: 10.1186/s13287-020-01627-5. Stem Cell Res Ther. 2020. PMID: 32138750 Free PMC article.
Cited by
-
Efficacy of stem cell therapy in patients with chronic liver disease: an umbrella review of systematic reviews.Int J Surg. 2024 Nov 1;110(11):6848-6861. doi: 10.1097/JS9.0000000000001644. Int J Surg. 2024. PMID: 38775499 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
