Nanomaterial-based bioorthogonal nanozymes for biological applications
- PMID: 34787131
- PMCID: PMC8862209
- DOI: 10.1039/d0cs00659a
Nanomaterial-based bioorthogonal nanozymes for biological applications
Abstract
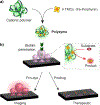
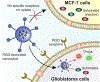
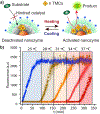
Bioorthogonal transformations are chemical reactions that use pathways which biological processes do not access. Bioorthogonal chemistry provides new approaches for imaging and therapeutic strategies, as well as tools for fundamental biology. Bioorthogonal catalysis enables the development of bioorthogonal "factories" for on-demand and in situ generation of drugs and imaging tools. Transition metal catalysts (TMCs) are widely employed as bioorthogonal catalysts due to their high efficiency and versatility. The direct application of TMCs in living systems is challenging, however, due to their limited solubility, instability in biological media and toxicity. Incorporation of TMCs into nanomaterial scaffolds can be used to enhance aqueous solubility, improve long-term stability in biological environment and minimize cytotoxicity. These nanomaterial platforms can be engineered for biomedical applications, increasing cellular uptake, directing biodistribution, and enabling active targeting. This review summarizes strategies for incorporating TMCs into nanomaterial scaffolds, demonstrating the potential and challenges of moving bioorthogonal nanocatalysts and nanozymes toward the clinic.
Conflict of interest statement
Conflicts of interest
There are no conflicts to declare.
Figures