Detection of SARS-CoV-2 through pool testing for COVID-19: an integrative review
- PMID: 34787261
- PMCID: PMC8582953
- DOI: 10.1590/0037-8682-0276-2021
Detection of SARS-CoV-2 through pool testing for COVID-19: an integrative review
Abstract
Introduction: The pool testing technique optimizes the number of tests performed and reduces the delivery time of results, which is an interesting strategy for the health crisis caused by the COVID-19 pandemic. This integrative review investigated studies in which pool testing was carried out for epidemiological or screening purposes to analyze its clinical or cost effectiveness and assessed the applicability of this method in high-, middle-, and low-income countries.
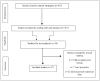
Methods: This integrative review used primary studies published in the MEDLINE, EMBASE, Literatura Latino-Americana e do Caribe em Ciências da Saúde (LILACS), and Cochrane Library databases.
Results: A total of 435 studies were identified: 35.3% were carried out in Asia, 29.4% in Europe, 29.4% in North America, and 5.9% in Oceania.
Conclusions: This review suggests that pool testing in the general population may be a useful surveillance strategy to detect new variants of SARS-CoV-2 and to evaluate the period of immunogenicity and global immunity from vaccines.
Conflict of interest statement
Conflict of Interest: The authors declare that there is no conflict of interest.
References
-
- Michelen M, Jones N, Stavropoulou C. In patients of COVID-19, What are the symptoms and clinical features of mild and moderate cases? University of Oxford; Apr 01, 2020. [2021 Jan 23]. The Center for Evidence-Based Medicine. Avaiable from: https://www.cebm.net/COVID-19/in-patients-of-COVID-19-what-are-the-sympt... .
-
- Lipsitch M, Swerdlow D L, Finelli L. Defining the epidemiology of COVID-19 - studies needed. N Engl J Med. 2020;382(13):1194–1196. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous