Immunogenicity and Safety of Investigational MenABCWY Vaccine and of 4CMenB and MenACWY Vaccines Administered Concomitantly or Alone: a Phase 2 Randomized Study of Adolescents and Young Adults
- PMID: 34787449
- PMCID: PMC8597725
- DOI: 10.1128/mSphere.00553-21
Immunogenicity and Safety of Investigational MenABCWY Vaccine and of 4CMenB and MenACWY Vaccines Administered Concomitantly or Alone: a Phase 2 Randomized Study of Adolescents and Young Adults
Abstract
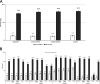
This phase 2, randomized, open-label study assessed the immunogenicity and safety of an investigational meningococcal ABCWY vaccine (MenABCWY) that contains components of licensed vaccines against meningococcal serogroup B (4CMenB) and serogroups ACWY (MenACWY). A total of 500 healthy 10- to 25-year-old participants were randomly assigned to one of five study groups in a 1:1:1:1:1 ratio. Four groups received two doses 2 months apart of MenABCWY and 4CMenB plus MenACWY administered concomitantly in the same arm (4CMenB+ACWY/S group) or different arms (4CMenB+ACWY/D group) or 4CMenB administered alone. A fifth group received a single MenACWY dose. Immunogenicity was determined by serum bactericidal assay using human complement (hSBA). The study was powered to assess immunological interference against pooled serogroup B test strains. One month after the second vaccine dose, hSBA geometric mean titers (GMTs) (with 80% confidence intervals [CI]) against pooled serogroup B strains were 31.84 (80% CI, 28.18 to 35.98), 38.48 (80% CI, 34.23 to 43.26), 40.08 (80% CI, 35.44 to 45.33), and 42.38 (80% CI, 37.31 to 48.13) in the MenABCWY, 4CMenB+ACWY/S, 4CMenB+ACWY/D, and 4CMenB groups, respectively. Immune responses (GMTs and 80% CIs) were lower for PorA and NHBA serogroup B test strains in the MenABCWY group compared to the 4CMenB+ACWY/D group and 4CMenB group. Evaluation of solicited and unsolicited adverse events (AEs) identified no safety concerns for the MenABCWY vaccine. One serious AE (syncope in the 4CMenB group) was considered related to vaccination. In conclusion, there is no evidence of substantial immunological interference between 4CMenB and MenACWY vaccine components against serogroup B. The safety and tolerability profile of the investigational MenABCWY vaccine was acceptable. (This study has been registered at ClinicalTrials.gov under registration no. NCT03587207.) IMPORTANCE The bacterial species Neisseria meningitidis is a major cause of meningitis, with six meningococcal groups (serogroups) causing most cases. A licensed vaccine, MenACWY (Menveo), targets four of these meningococcal serogroups, and another vaccine, 4CMenB (Bexsero), targets serogroup B. A combined vaccine (MenABCWY) that targets all five serogroups is under development to simplify the vaccination schedule. In a previous study, the immune response to serogroup B was found to be overall higher in individuals who received 4CMenB than in those who received an investigational MenABCWY vaccine. We investigated this further by giving healthy adolescents and young adults the MenABCWY vaccine, 4CMenB plus MenACWY vaccine in the same or different arms, 4CMenB vaccine alone, or MenACWY vaccine alone. Immunogenicity results for serogroup B across study groups suggest no major interference between the MenB and MenACWY vaccine components. This supports further development of the combined MenABCWY vaccine.
Keywords: MenABCWY; Neisseria meningitidis; immunogenicity; investigational.
Figures


References
-
- Acevedo R, Bai X, Borrow R, Caugant DA, Carlos J, Ceyhan M, Christensen H, Climent Y, De Wals P, Dinleyici EC, Echaniz-Aviles G, Hakawi A, Kamiya H, Karachaliou A, Lucidarme J, Meiring S, Mironov K, Safadi MAP, Shao Z, Smith V, Steffen R, Stenmark B, Taha MK, Trotter C, Vazquez JA, Zhu B. 2019. The Global Meningococcal Initiative meeting on prevention of meningococcal disease worldwide: epidemiology, surveillance, hypervirulent strains, antibiotic resistance and high-risk populations. Expert Rev Vaccines 18:15–30. doi: 10.1080/14760584.2019.1557520. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
