A Smoking Cessation App for Nondaily Smokers (Version 2 of the Smiling Instead of Smoking App): Acceptability and Feasibility Study
- PMID: 34787577
- PMCID: PMC8663587
- DOI: 10.2196/29760
A Smoking Cessation App for Nondaily Smokers (Version 2 of the Smiling Instead of Smoking App): Acceptability and Feasibility Study
Abstract
Background: Recent evidence highlights the significant detrimental impact of nondaily smoking on health and its disproportionate prevalence in underserved populations; however, little work has been done to develop treatments specifically geared toward quitting nondaily smoking.
Objective: This study aims to test the feasibility, acceptability, and conceptual underpinnings of version 2 of the Smiling Instead of Smoking (SiS2) smartphone app, which was developed specifically for nondaily smokers and uses a positive psychology approach.
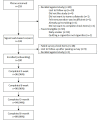
Methods: In a prospective, single-group study, nondaily smokers (N=100) were prescribed use of the SiS2 app for 7 weeks while undergoing a quit attempt. The app assigned daily positive psychology exercises and behavioral tasks every 2 to 3 days, which guided smokers through using the smoking cessation tools offered in the app. Participants answered surveys at baseline and at 2, 6, 12, and 24 weeks postquit. Feasibility was evaluated based on app use and acceptability based on survey responses. The underlying conceptual framework was tested by examining whether theorized within-person changes occurred from baseline to end of treatment on scales measuring self-efficacy, desire to smoke, and processing of self-relevant health information (ie, pros and cons of smoking, importance of the pros and cons of quitting, and motivation).
Results: Participants used the SiS2 app on an average of 24.7 (SD 13.8) days out of the 49 prescribed days. At the end of treatment, most participants rated the functions of the app as very easy to use (eg, 70/95, 74% regarding cigarette log and 59/95, 62% regarding happiness exercises). The average score on the System Usability Scale was 79.8 (SD 17.3; A grade; A+ ≥84.1, B+ <78.8). Most participants reported that the app helped them in their quit attempt (83/95, 87%), and helped them stay positive while quitting (78/95, 82%). Large effects were found for within-person decreases in the desire to smoke (b=-1.5, 95% CI -1.9 to -1.1; P<.001; gav=1.01), the importance of the pros of smoking (b=-20.7, 95% CI -27.2 to -14.3; P<.001; gav=0.83), and perceived psychoactive benefits of smoking (b=-0.8, 95% CI -1.0 to -0.5; P<.001; gav=0.80). Medium effects were found for increases in self-efficacy for remaining abstinent when encountering internal (b=13.1, 95% CI 7.6 to 18.7; P<.001; gav=0.53) and external (b=11.2, 95% CI 6.1 to 16.1; P<.001; gav=0.49) smoking cues. Smaller effects, contrary to expectations, were found for decreases in motivation to quit smoking (P=.005) and the perceived importance of the pros of quitting (P=.009). Self-reported 30-day point prevalence abstinence rates were 40%, 56%, and 56% at 6, 12, and 24 weeks after the quit day, respectively.
Conclusions: The SiS2 app was feasible and acceptable, showed promising changes in constructs relevant to smoking cessation, and had high self-reported quit rates by nondaily smokers. The SiS2 app warrants testing in a randomized controlled trial.
Keywords: happiness; mHealth; mobile phone; nondaily; positive psychology; smartphone app; smoking cessation.
©Bettina B Hoeppner, Kaitlyn R Siegel, Hannah A Carlon, Christopher W Kahler, Elyse R Park, Susanne S Hoeppner. Originally published in JMIR Formative Research (https://formative.jmir.org), 17.11.2021.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures


Similar articles
-
Testing the Outcomes of a Smoking Cessation Smartphone App for Nondaily Smokers: Protocol for a Proof-of-concept Randomized Controlled Trial.JMIR Res Protoc. 2023 Feb 14;12:e40867. doi: 10.2196/40867. JMIR Res Protoc. 2023. PMID: 36787172 Free PMC article.
-
Feature-Level Analysis of a Smoking Cessation Smartphone App Based on a Positive Psychology Approach: Prospective Observational Study.JMIR Form Res. 2022 Jul 28;6(7):e38234. doi: 10.2196/38234. JMIR Form Res. 2022. PMID: 35900835 Free PMC article.
-
Smoking Cessation Smartphone App for Nondaily Smoking With Telephone Onboarding: Proof-of-Concept Randomized Controlled Trial.JMIR Mhealth Uhealth. 2025 Jan 15;13:e53971. doi: 10.2196/53971. JMIR Mhealth Uhealth. 2025. PMID: 39814363 Free PMC article. Clinical Trial.
-
Leveraging Positive Psychology to Support Smoking Cessation in Nondaily Smokers Using a Smartphone App: Feasibility and Acceptability Study.JMIR Mhealth Uhealth. 2019 Jul 3;7(7):e13436. doi: 10.2196/13436. JMIR Mhealth Uhealth. 2019. PMID: 31271147 Free PMC article.
-
Treating Smoking in Cancer Patients: An Essential Component of Cancer Care [Internet].Bethesda (MD): National Cancer Institute (US); 2022 Jun. Bethesda (MD): National Cancer Institute (US); 2022 Jun. PMID: 36049036 Free Books & Documents. Review.
Cited by
-
Prevalence and Trends in Cigarette Smoking With and Without Tobacco Use Disorder Among Adults in the United States: 2010-2021.J Clin Psychiatry. 2024 Jun 12;85(3):23m15086. doi: 10.4088/JCP.23m15086. J Clin Psychiatry. 2024. PMID: 38874573 Free PMC article.
-
Testing the Outcomes of a Smoking Cessation Smartphone App for Nondaily Smokers: Protocol for a Proof-of-concept Randomized Controlled Trial.JMIR Res Protoc. 2023 Feb 14;12:e40867. doi: 10.2196/40867. JMIR Res Protoc. 2023. PMID: 36787172 Free PMC article.
-
Feature-Level Analysis of a Smoking Cessation Smartphone App Based on a Positive Psychology Approach: Prospective Observational Study.JMIR Form Res. 2022 Jul 28;6(7):e38234. doi: 10.2196/38234. JMIR Form Res. 2022. PMID: 35900835 Free PMC article.
-
Smoking Cessation Smartphone App for Nondaily Smoking With Telephone Onboarding: Proof-of-Concept Randomized Controlled Trial.JMIR Mhealth Uhealth. 2025 Jan 15;13:e53971. doi: 10.2196/53971. JMIR Mhealth Uhealth. 2025. PMID: 39814363 Free PMC article. Clinical Trial.
-
A secondary analysis examining the performance of the State Optimism Measure (SOM) compared to the Life Orientation Test-Revised (LOT-R) in measuring optimism over time.Psychol Health. 2024 Jul;39(7):989-1004. doi: 10.1080/08870446.2022.2126472. Epub 2022 Sep 26. Psychol Health. 2024. PMID: 36154764 Free PMC article.
References
-
- Centers for Disease ControlPrevention (CDC) Cigarette smoking among adults and trends in smoking cessation - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009 Nov 13;58(44):1227–32. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5844a2.htm mm5844a2 - PubMed
-
- National Cancer Institute What's in a name? Examination of light and intermittent smokers (NIH Publication) US Department of Health and Human Services. 2007. [2021-09-22]. https://scholar.google.com/scholar?cluster=13594314118933346903&hl=en&as... .
LinkOut - more resources
Full Text Sources
Miscellaneous

