Inhaled Pulmonary Vasodilator Therapy in Adult Lung Transplant: A Randomized Clinical Trial
- PMID: 34787647
- PMCID: PMC8600451
- DOI: 10.1001/jamasurg.2021.5856
Inhaled Pulmonary Vasodilator Therapy in Adult Lung Transplant: A Randomized Clinical Trial
Abstract
Importance: Inhaled nitric oxide (iNO) is commonly administered for selectively inhaled pulmonary vasodilation and prevention of oxidative injury after lung transplant (LT). Inhaled epoprostenol (iEPO) has been introduced worldwide as a cost-saving alternative to iNO without high-grade evidence for this indication.
Objective: To investigate whether the use of iEPO will lead to similar rates of severe/grade 3 primary graft dysfunction (PGD-3) after adult LT when compared with use of iNO.
Design, setting, and participants: This health system-funded, randomized, blinded (to participants, clinicians, data managers, and the statistician), parallel-designed, equivalence clinical trial included 201 adult patients who underwent single or bilateral LT between May 30, 2017, and March 21, 2020. Patients were grouped into 5 strata according to key prognostic clinical features and randomized per stratum to receive either iNO or iEPO at the time of LT via 1:1 treatment allocation.
Interventions: Treatment with iNO or iEPO initiated in the operating room before lung allograft reperfusion and administered continously until cessation criteria met in the intensive care unit (ICU).
Main outcomes and measures: The primary outcome was PGD-3 development at 24, 48, or 72 hours after LT. The primary analysis was for equivalence using a two one-sided test (TOST) procedure (90% CI) with a margin of 19% for between-group PGD-3 risk difference. Secondary outcomes included duration of mechanical ventilation, hospital and ICU lengths of stay, incidence and severity of acute kidney injury, postoperative tracheostomy placement, and in-hospital, 30-day, and 90-day mortality rates. An intention-to-treat analysis was performed for the primary and secondary outcomes, supplemented by per-protocol analysis for the primary outcome.
Results: A total of 201 randomized patients met eligibility criteria at the time of LT (129 men [64.2%]). In the intention-to-treat population, 103 patients received iEPO and 98 received iNO. The primary outcome occurred in 46 of 103 patients (44.7%) in the iEPO group and 39 of 98 (39.8%) in the iNO group, leading to a risk difference of 4.9% (TOST 90% CI, -6.4% to 16.2%; P = .02 for equivalence). There were no significant between-group differences for secondary outcomes.
Conclusions and relevance: Among patients undergoing LT, use of iEPO was associated with similar risks for PGD-3 development and other postoperative outcomes compared with the use of iNO.
Trial registration: ClinicalTrials.gov identifier: NCT03081052.
Conflict of interest statement
Figures

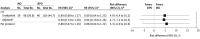
Comment in
-
Role of Pulmonary Vasodilators in Ameliorating Primary Graft Dysfunction Following Lung Transplant.JAMA Surg. 2022 Jan 1;157(1):e215857. doi: 10.1001/jamasurg.2021.5857. Epub 2022 Jan 12. JAMA Surg. 2022. PMID: 34787654 Free PMC article. No abstract available.
References
-
- Shargall Y, Guenther G, Ahya VN, Ardehali A, Singhal A, Keshavjee S; ISHLT Working Group on Primary Lung Graft Dysfunction . Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24(10):1489-1500. doi:10.1016/j.healun.2005.03.011 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

