Volumetric morphometry reveals spindle width as the best predictor of mammalian spindle scaling
- PMID: 34787651
- PMCID: PMC8719715
- DOI: 10.1083/jcb.202106170
Volumetric morphometry reveals spindle width as the best predictor of mammalian spindle scaling
Abstract
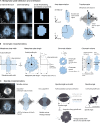
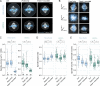
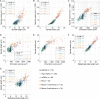
The function of cellular structures at the mesoscale is dependent on their geometry and proportionality to cell size. The mitotic spindle is a good example why length and shape of intracellular organelles matter. Spindle length determines the distance over which chromosomes will segregate, and spindle shape ensures bipolarity. While we still lack a systematic and quantitative understanding of subcellular morphology, new imaging techniques and volumetric data analysis promise novel insights into scaling relations across different species. Here, we introduce Spindle3D, an open-source plug-in that allows for the quantitative, consistent, and automated analysis of 3D fluorescent data of spindles and chromatin. We systematically analyze different mammalian cell types, including somatic cells, stem cells, and one- and two-cell embryos, to derive volumetric relations of spindle, chromatin, and the cell. Taken together, our data indicate that mitotic spindle width is a robust indicator of spindle volume, which correlates linearly with chromatin and cell volume both within single cell types and across mammalian species.
© 2021 Kletter et al.
Figures