Immunogenicity of Anti-SARS-CoV-2 Vaccines in Common Variable Immunodeficiency
- PMID: 34787773
- PMCID: PMC8596355
- DOI: 10.1007/s10875-021-01174-5
Immunogenicity of Anti-SARS-CoV-2 Vaccines in Common Variable Immunodeficiency
Abstract
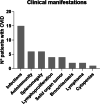
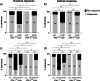
Common variable immunodeficiency (CVID) is characterized by hypogammaglobulinemia and/or a defective antibody response to T-dependent and T-independent antigens. CVID response to immunization depends on the antigen type, the vaccine mechanism, and the specific patient immune defect. In CVID patients, humoral and cellular responses to the currently used COVID-19 vaccines remain unexplored. Eighteen CVID subjects receiving 2-dose anti-SARS-CoV-2 vaccines were prospectively studied. S1-antibodies and S1-specific IFN-γ T cell response were determined by ELISA and FluoroSpot, respectively. The immune response was measured before the administration and after each dose of the vaccine, and it was compared to the response of 50 healthy controls (HC). The development of humoral and cellular responses was slower in CVID patients compared with HC. After completing vaccination, 83% of CVID patients had S1-specific antibodies and 83% had S1-specific T cells compared with 100% and 98% of HC (p = 0.014 and p = 0.062, respectively), but neutralizing antibodies were detected only in 50% of the patients. The strength of both humoral and cellular responses was significantly lower in CVID compared with HC, after the first and second doses of the vaccine. Absent or discordant humoral and cellular responses were associated with previous history of autoimmunity and/or lymphoproliferation. Among the three patients lacking humoral response, two had received recent therapy with anti-B cell antibodies. Further studies are needed to understand if the response to COVID-19 vaccination in CVID patients is protective enough. The 2-dose vaccine schedule and possibly a third dose might be especially necessary to achieve full immune response in these patients.
Keywords: COVID-19; Common variable immunodeficiency; Immunogenicity; Primary immunodeficiency diseases; SARS-CoV-2; Vaccination.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Characterization of SARS-CoV-2-Specific Humoral and Cellular Immune Responses Induced by Inactivated COVID-19 Vaccines in a Real-World Setting.Front Immunol. 2021 Dec 22;12:802858. doi: 10.3389/fimmu.2021.802858. eCollection 2021. Front Immunol. 2021. PMID: 35003131 Free PMC article.
-
Cellular and humoral immunogenicity of a SARS-CoV-2 mRNA vaccine in patients on haemodialysis.EBioMedicine. 2021 Aug;70:103524. doi: 10.1016/j.ebiom.2021.103524. Epub 2021 Aug 12. EBioMedicine. 2021. PMID: 34391096 Free PMC article.
-
The Immune Response to SARS-CoV-2 Vaccination: Insights Learned From Adult Patients With Common Variable Immune Deficiency.Front Immunol. 2022 Jan 19;12:815404. doi: 10.3389/fimmu.2021.815404. eCollection 2021. Front Immunol. 2022. PMID: 35126372 Free PMC article.
-
Easy approach to detect cell immunity to COVID vaccines in common variable immunodeficiency patients.Allergol Immunopathol (Madr). 2022 May 1;50(3):101-105. doi: 10.15586/aei.v50i3.583. eCollection 2022. Allergol Immunopathol (Madr). 2022. PMID: 35527662 Review.
-
Immunogenicity and risk factors for poor humoral immune response to SARS-CoV-2 vaccine in patients with autoimmune hepatitis: a systematic review and meta-analysis.Rev Esp Enferm Dig. 2024 Dec;116(12):671-679. doi: 10.17235/reed.2024.10053/2023. Rev Esp Enferm Dig. 2024. PMID: 38235657
Cited by
-
Antibody response following the third and fourth SARS-CoV-2 vaccine dose in individuals with common variable immunodeficiency.Front Immunol. 2022 Jul 28;13:934476. doi: 10.3389/fimmu.2022.934476. eCollection 2022. Front Immunol. 2022. PMID: 35967433 Free PMC article.
-
Impact of Exposure to Vaccination and Infection on Cellular and Antibody Response to SARS-CoV-2 in CVID Patients Through COVID-19 Pandemic.J Clin Immunol. 2023 Dec 22;44(1):12. doi: 10.1007/s10875-023-01616-2. J Clin Immunol. 2023. PMID: 38129351
-
SARS-CoV-2 T Cell Response in Severe and Fatal COVID-19 in Primary Antibody Deficiency Patients Without Specific Humoral Immunity.Front Immunol. 2022 Mar 10;13:840126. doi: 10.3389/fimmu.2022.840126. eCollection 2022. Front Immunol. 2022. PMID: 35359967 Free PMC article.
-
Adaptive Cellular Responses following SARS-CoV-2 Vaccination in Primary Antibody Deficiency Patients.Pathogens. 2024 Jun 18;13(6):514. doi: 10.3390/pathogens13060514. Pathogens. 2024. PMID: 38921811 Free PMC article.
-
Detection of specific RBD+ IgG+ memory B cells by flow cytometry in healthcare workers and patients with inborn errors of immunity after BNT162b2 m RNA COVID-19 vaccination.Front Immunol. 2023 May 4;14:1136308. doi: 10.3389/fimmu.2023.1136308. eCollection 2023. Front Immunol. 2023. PMID: 37215146 Free PMC article.
References
-
- Seidel MG, Kindle G, Gathmann B, Quinti I, Buckland M, van Montfrans J, et al. The European Society for Immunodeficiencies (ESID) Registry Working Definitions for the Clinical Diagnosis of Inborn Errors of Immunity. J Allergy Clin Immunol Pract. 2019;7:1763–1770. doi: 10.1016/j.jaip.2019.02.004. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

