Application of Human Epineural Conduit Supported with Human Mesenchymal Stem Cells as a Novel Therapy for Enhancement of Nerve Gap Regeneration
- PMID: 34787795
- PMCID: PMC8930890
- DOI: 10.1007/s12015-021-10301-z
Application of Human Epineural Conduit Supported with Human Mesenchymal Stem Cells as a Novel Therapy for Enhancement of Nerve Gap Regeneration
Abstract
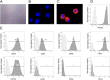
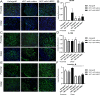
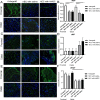
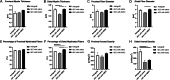
Various therapeutic methods have been suggested to enhance nerve regeneration. In this study, we propose a novel approach for enhancement of nerve gap regeneration by applying human epineural conduit (hEC) supported with human mesenchymal stem cells (hMSC), as an alternative to autograft repair. Restoration of 20 mm sciatic nerve defect with hEC created from human sciatic nerve supported with hMSC was tested in 4 experimental groups (n = 6 each) in the athymic nude rat model (Crl:NIH-Foxn1rnu): 1 - No repair control, 2 - Autograft control, 3 - Matched diameter hEC filled with 1 mL saline, 4 - Matched diameter hEC supported with 3 × 106 hMSC. Assessments included: functional tests: toe-spread and pinprick, regeneration assessment by immunofluorescence staining: HLA-1, HLA-DR, NGF, GFAP, Laminin B, S-100, VEGF, vWF and PKH26 labeling; histomorphometric analysis of myelin thickness, axonal density, fiber diameter and myelinated nerve fibers percentage; Gastrocnemius Muscle Index (GMI) and muscle fiber area ratio. Best sensory and motor function recovery, as well as GMI and muscle fiber area ratio, were observed in the autograft group, and were comparable to the hEC with hMSC group (p = 0.038). Significant improvements of myelin thickness (p = 0.003), fiber diameter (p = 0.0296), and percentage of myelinated fibers (p < 0.0001) were detected in hEC group supported with hMSC compared to hEC with saline controls. At 12-weeks after nerve gap repair, hEC combined with hMSC revealed increased expression of neurotrophic and proangiogenic factors, which corresponded with improvement of function comparable with the autograft control. Application of our novel hEC supported with hMSC provides a potential alternative to the autograft nerve repair.
Keywords: Autograft; Human Epineural conduit; Mesenchymal stem cells; Nerve regeneration; Peripheral nerve repair; Regenerative medicine.
© 2021. The Author(s).
Conflict of interest statement
M.S. is the inventor of the patent application related to Methods of Engineering of Neural Tissue (US/2012/171172A1) and holds a patent on the use of epineural sheath grafts for neural regeneration and protection (WO/2009/124170A1). The authors M.S., M.M.S., K.K., S.B., W.G.K., and J.C. do not have any non-financial conflict of interest.
Figures




References
-
- Noble J, Munro C, Prasad V, Midha R. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. The Journal of Trauma. 1998;45(1):116–122. - PubMed
-
- Kouyoumdjian JA, Graça CR, FMF V. Peripheral nerve injuries: A retrospective survey of 1124 cases. Neurology India. 2017;65(3):551–555. - PubMed
-
- Kelsey, J.L., Praemer, A., Nelson, L.M., Felberg, A., Rice, D.P. (1997). Upper extremity disorders: Frequency, impact and cost. New York, Churchill Livingstone, p.26–42.
-
- Campbell WW. Evaluation and management of peripheral nerve injury. Clinical Neurophysiology. 2008;119(9):1951–1965. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

