Retention of the NLRP3 Inflammasome-Primed Neutrophils in the Bone Marrow Is Essential for Myocardial Infarction-Induced Granulopoiesis
- PMID: 34788059
- PMCID: PMC8716427
- DOI: 10.1161/CIRCULATIONAHA.121.056019
Retention of the NLRP3 Inflammasome-Primed Neutrophils in the Bone Marrow Is Essential for Myocardial Infarction-Induced Granulopoiesis
Abstract
Background: Acute myocardial infarction (MI) results in overzealous production and infiltration of neutrophils to the ischemic heart. This is mediated in part by granulopoiesis induced by the S100A8/A9-NLRP3-IL-1β signaling axis in injury-exposed neutrophils. Despite the transcriptional upregulation of the NLRP3 (Nod Like Receptor Family Pyrin Domain-Containing 3) inflammasome and associated signaling components in neutrophils, the serum levels of IL-1β (interleukin-1β), the effector molecule in granulopoiesis, were not affected by MI, suggesting that IL-1β is not released systemically. We hypothesize that IL-1β is released locally within the bone marrow (BM) by inflammasome-primed and reverse-migrating neutrophils.
Methods: Using a combination of time-dependent parabiosis and flow cytometry techniques, we first characterized the migration patterns of different blood cell types across the parabiotic barrier. We next induced MI in parabiotic mice by permanent ligation of the left anterior descending artery and examined the ability of injury-exposed neutrophils to permeate the parabiotic barrier and induce granulopoiesis in noninfarcted parabionts. Last, using multiple neutrophil adoptive and BM transplant studies, we studied the molecular mechanisms that govern reverse migration and retention of the primed neutrophils, IL-1β secretion, and granulopoiesis. Cardiac function was assessed by echocardiography.
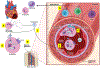
Results: MI promoted greater accumulation of the inflammasome-primed neutrophils in the BM. Introducing a time-dependent parabiotic barrier to the free movement of neutrophils inhibited their ability to stimulate granulopoiesis in the noninfarcted parabionts. Previous priming of the NLRP3 inflammasome is not a prerequisite, but the presence of a functional CXCR4 (C-X-C-motif chemokine receptor 4) on the primed-neutrophils and elevated serum S100A8/A9 levels are necessary for homing and retention of the reverse-migrating neutrophils. In the BM, the primed-neutrophils secrete IL-1β through formation of gasdermin D pores and promote granulopoiesis. Pharmacological and genetic strategies aimed at the inhibition of neutrophil homing or release of IL-1β in the BM markedly suppressed MI-induced granulopoiesis and improved cardiac function.
Conclusions: Our data reveal a new paradigm of how circulatory cells establish a direct communication between organs by delivering signaling molecules (eg, IL-1β) directly at the sites of action rather through systemic release. We suggest that this pathway may exist to limit the off-target effects of systemic IL-1β release.
Keywords: NLRP3 inflammasome; S100A8/A9; granulopoiesis; interleukin-1beta; leukocytosis; myocardial infarction; neutrophils.
Figures

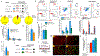



Comment in
-
Letter by Ren and Yang Regarding Article, "Retention of the NLRP3 Inflammasome-Primed Neutrophils in the Bone Marrow Is Essential for Myocardial Infarction-Induced Granulopoiesis".Circulation. 2022 May 10;145(19):e1034. doi: 10.1161/CIRCULATIONAHA.122.059645. Epub 2022 May 9. Circulation. 2022. PMID: 35533215 No abstract available.
-
Response by Nagareddy and Sreejit to Letter Regarding Article, "Retention of the NLRP3 Inflammasome-Primed Neutrophils in the Bone Marrow Is Essential for Myocardial Infarction-Induced Granulopoiesis".Circulation. 2022 May 10;145(19):e1035-e1036. doi: 10.1161/CIRCULATIONAHA.122.059691. Epub 2022 May 9. Circulation. 2022. PMID: 35533219 Free PMC article. No abstract available.
References
-
- Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–3047. doi: 10.1084/jem.20070885. - DOI - PMC - PubMed
-
- Horckmans M, Ring L, Duchene J, Santovito D, Schloss MJ, Drechsler M, Weber C, Soehnlein O, Steffens S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. European heart journal. 2017;38:187–197. doi: 10.1093/eurheartj/ehw002. - DOI - PubMed
-
- O’Donoghue M, Morrow DA, Cannon CP, Guo W, Murphy SA, Gibson CM, Sabatine MS. Association between baseline neutrophil count, clopidogrel therapy, and clinical and angiographic outcomes in patients with ST-elevation myocardial infarction receiving fibrinolytic therapy. European heart journal. 2008;29:984–991. doi: 10.1093/eurheartj/ehn112. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

