Localized drug delivery graphene bioscaffolds for cotransplantation of islets and mesenchymal stem cells
- PMID: 34788097
- PMCID: PMC8597999
- DOI: 10.1126/sciadv.abf9221
Localized drug delivery graphene bioscaffolds for cotransplantation of islets and mesenchymal stem cells
Abstract
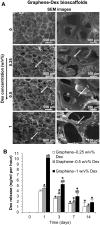
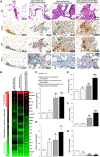
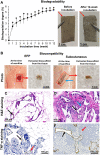
In the present work, we developed, characterized, and tested an implantable graphene bioscaffold which elutes dexamethasone (Dex) that can accommodate islets and adipose tissue–derived mesenchymal stem cells (AD-MSCs). In vitro studies demonstrated that islets in graphene–0.5 w/v% Dex bioscaffolds had a substantial higher viability and function compared to islets in graphene-alone bioscaffolds or islets cultured alone (P < 0.05). In vivo studies, in which bioscaffolds were transplanted into the epididymal fat pad of diabetic mice, demonstrated that, when islet:AD-MSC units were seeded into graphene–0.5 w/v% Dex bioscaffolds, this resulted in complete restoration of glycemic control immediately after transplantation with these islets also showing a faster response to glucose challenges (P < 0.05). Hence, this combination approach of using a graphene bioscaffold that can be functionalized for local delivery of Dex into the surrounding microenvironment, together with AD-MSC therapy, can significantly improve the survival and function of transplanted islets.
Figures



References
-
- Pellegrini S., Cantarelli E., Sordi V., Nano R., Piemonti L., The state of the art of islet transplantation and cell therapy in type 1 diabetes. Acta Diabetol. 53, 683–691 (2016). - PubMed
-
- Moberg L., The role of the innate immunity in islet transplantation. Ups. J. Med. Sci. 110, 17–56 (2005). - PubMed
-
- Shapiro A. J., Ricordi C., Hering B., Edmonton’s islet success has indeed been replicated elsewhere. The Lancet 362, 1242 (2003). - PubMed
-
- Rajab A., Islet transplantation: Alternative sites. Curr. Diab. Rep. 10, 332–337 (2010). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

