Evaluation of the United States COVID-19 vaccine allocation strategy
- PMID: 34788345
- PMCID: PMC8598051
- DOI: 10.1371/journal.pone.0259700
Evaluation of the United States COVID-19 vaccine allocation strategy
Abstract
Background: Anticipating an initial shortage of vaccines for COVID-19, the Centers for Disease Control (CDC) in the United States developed priority vaccine allocations for specific demographic groups in the population. This study evaluates the performance of the CDC vaccine allocation strategy with respect to multiple potentially competing vaccination goals (minimizing mortality, cases, infections, and years of life lost (YLL)), under the same framework as the CDC allocation: four priority vaccination groups and population demographics stratified by age, comorbidities, occupation and living condition (congested or non-congested).
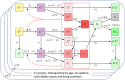
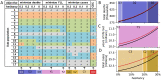
Methods and findings: We developed a compartmental disease model that incorporates key elements of the current pandemic including age-varying susceptibility to infection, age-varying clinical fraction, an active case-count dependent social distancing level, and time-varying infectivity (accounting for the emergence of more infectious virus strains). The CDC allocation strategy is compared to all other possibly optimal allocations that stagger vaccine roll-out in up to four phases (17.5 million strategies). The CDC allocation strategy performed well in all vaccination goals but never optimally. Under the developed model, the CDC allocation deviated from the optimal allocations by small amounts, with 0.19% more deaths, 4.0% more cases, 4.07% more infections, and 0.97% higher YLL, than the respective optimal strategies. The CDC decision to not prioritize the vaccination of individuals under the age of 16 was optimal, as was the prioritization of health-care workers and other essential workers over non-essential workers. Finally, a higher prioritization of individuals with comorbidities in all age groups improved outcomes compared to the CDC allocation.
Conclusion: The developed approach can be used to inform the design of future vaccine allocation strategies in the United States, or adapted for use by other countries seeking to optimize the effectiveness of their vaccine allocation strategies.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Dooling K, McClung N, Chamberland M, Marin M, Wallace M, Bell BP, et al.. The Advisory Committee on Immunization Practices’ Updated Interim Recommendation for Allocating Initial Supplies of COVID-19 Vaccine—United States, 2020. Morbidity and Mortality Weekly Report. 2021;69(51-52):1657–1660. doi: 10.15585/mmwr.mm695152e2 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

