Multiyear Factor VIII Expression after AAV Gene Transfer for Hemophilia A
- PMID: 34788507
- PMCID: PMC8672712
- DOI: 10.1056/NEJMoa2104205
Multiyear Factor VIII Expression after AAV Gene Transfer for Hemophilia A
Abstract
Background: The goal of gene therapy for patients with hemophilia A is to safely impart long-term stable factor VIII expression that predictably ameliorates bleeding with the use of the lowest possible vector dose.
Methods: In this phase 1-2 trial, we infused an investigational adeno-associated viral (AAV) vector (SPK-8011) for hepatocyte expression of factor VIII in 18 men with hemophilia A. Four dose cohorts were enrolled; the lowest-dose cohort received a dose of 5 × 1011 vector genomes (vg) per kilogram of body weight, and the highest-dose cohort received 2 × 1012 vg per kilogram. Some participants received glucocorticoids within 52 weeks after vector administration either to prevent or to treat a presumed AAV capsid immune response. Trial objectives included evaluation of the safety and preliminary efficacy of SPK-8011 and of the expression and durability of factor VIII.
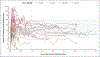
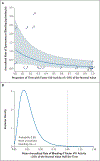
Results: The median safety observation period was 36.6 months (range, 5.5 to 50.3). A total of 33 treatment-related adverse events occurred in 8 participants; 17 events were vector-related, including 1 serious adverse event, and 16 were glucocorticoid-related. Two participants lost all factor VIII expression because of an anti-AAV capsid cellular immune response that was not sensitive to immune suppression. In the remaining 16 participants, factor VIII expression was maintained; 12 of these participants were followed for more than 2 years, and a one-stage factor VIII assay showed no apparent decrease in factor VIII activity over time (mean [±SD] factor VIII activity, 12.9±6.9% of the normal value at 26 to 52 weeks when the participants were not receiving glucocorticoids vs. 12.0±7.1% of the normal value at >52 weeks after vector administration; 95% confidence interval [CI], -2.4 to 0.6 for the difference between matched pairs). The participants had a 91.5% reduction (95% CI, 88.8 to 94.1) in the annualized bleeding rate (median rate, 8.5 events per year [range, 0 to 43.0] before vector administration vs. 0.3 events per year [range, 0 to 6.5] after vector administration).
Conclusions: Sustained factor VIII expression in 16 of 18 participants who received SPK-8011 permitted discontinuation of prophylaxis and a reduction in bleeding episodes. No major safety concerns were reported. (Funded by Spark Therapeutics and the National Heart, Lung, and Blood Institute; ClinicalTrials.gov numbers, NCT03003533 and NCT03432520.).
Copyright © 2021 Massachusetts Medical Society.
Figures

References
-
- Mannucci PM, Tuddenham EG. The hemophilias — from royal genes to gene therapy. N Engl J Med 2001; 344: 1773–9. - PubMed
-
- den Uijl IEM, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia 2011; 17: 41–4. - PubMed
-
- Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med 2007; 357: 535–44. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
