ADH5-mediated NO bioactivity maintains metabolic homeostasis in brown adipose tissue
- PMID: 34788615
- PMCID: PMC8640996
- DOI: 10.1016/j.celrep.2021.110003
ADH5-mediated NO bioactivity maintains metabolic homeostasis in brown adipose tissue
Abstract
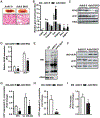
Brown adipose tissue (BAT) thermogenic activity is tightly regulated by cellular redox status, but the underlying molecular mechanisms are incompletely understood. Protein S-nitrosylation, the nitric-oxide-mediated cysteine thiol protein modification, plays important roles in cellular redox regulation. Here we show that diet-induced obesity (DIO) and acute cold exposure elevate BAT protein S-nitrosylation, including UCP1. This thermogenic-induced nitric oxide bioactivity is regulated by S-nitrosoglutathione reductase (GSNOR; alcohol dehydrogenase 5 [ADH5]), a denitrosylase that balances the intracellular nitroso-redox status. Loss of ADH5 in BAT impairs cold-induced UCP1-dependent thermogenesis and worsens obesity-associated metabolic dysfunction. Mechanistically, we demonstrate that Adh5 expression is induced by the transcription factor heat shock factor 1 (HSF1), and administration of an HSF1 activator to BAT of DIO mice increases Adh5 expression and significantly improves UCP1-mediated respiration. Together, these data indicate that ADH5 controls BAT nitroso-redox homeostasis to regulate adipose thermogenesis, which may be therapeutically targeted to improve metabolic health.
Keywords: ADH5; BAT; HSF1; alcohol dehydrogenase 5; brown adipose tissue; heat shock factor 1; nitrosative stress; obesity.
Copyright © 2021 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

