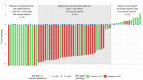
A third anti-SARS-CoV-2 mRNA dose does not overcome the pejorative impact of anti-CD20 therapy and/or low immunoglobulin levels in patients with lymphoma or chronic lymphocytic leukemia
- PMID: 34788987
- PMCID: PMC9152953
- DOI: 10.3324/haematol.2021.280026
A third anti-SARS-CoV-2 mRNA dose does not overcome the pejorative impact of anti-CD20 therapy and/or low immunoglobulin levels in patients with lymphoma or chronic lymphocytic leukemia
Figures

Comment in
-
When timing is more important than quantity in COVID-19 vaccination.Haematologica. 2022 Jun 1;107(6):1237-1238. doi: 10.3324/haematol.2021.280264. Haematologica. 2022. PMID: 34788989 Free PMC article. No abstract available.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

