Effect of Deferoxamine on Outcome According to Baseline Hematoma Volume: A Post Hoc Analysis of the i-DEF Trial
- PMID: 34789008
- PMCID: PMC8960321
- DOI: 10.1161/STROKEAHA.121.035421
Effect of Deferoxamine on Outcome According to Baseline Hematoma Volume: A Post Hoc Analysis of the i-DEF Trial
Abstract
Background: Hematoma volume (HV) is a powerful determinant of outcome after intracerebral hemorrhage. We examined whether the effect of the iron chelator, deferoxamine, on functional outcome varied depending on HV in the i-DEF trial (Intracerebral Hemorrhage Deferoxamine).
Methods: A post hoc analysis of the i-DEF trial; participants were classified according to baseline HV (small <10 mL, moderate 10-30 mL, and large >30 mL). Favorable outcome was defined as a modified Rankin Scale score of 0-2 at day-180; secondarily at day-90. Logistic regression was used to evaluate the differential treatment effect according to HV.
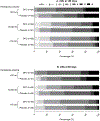
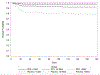
Results: Two hundred ninety-one subjects were included in the as-treated analysis; 121 with small, 114 moderate, and 56 large HV. Day-180 modified Rankin Scale scores were available for 270/291 subjects (111 with small, 105 moderate, and 54 large HV). There was a differential effect of treatment according to HV on day-180 outcomes (P-for-interaction =0.0077); 50% (27/54) of deferoxamine-treated patients with moderate HV had favorable outcome compared with 25.5% (13/51) of placebo-treated subjects (adjusted odds ratio, 2.7 [95% CI, 1.13-6.27]; P=0.0258). Treatment effect was not significant for small (adjusted odds ratio, 1.37 [95% CI, 0.62-3.02]) or large (adjusted odds ratio, 0.12 [95% CI, 0.01-1.05]) HV. Results for day-90 outcomes were comparable (P-for-interaction =0.0617). Sensitivity analyses yielded similar results.
Conclusions: Among patients with moderate HV, a greater proportion of deferoxamine- than placebo-treated patients achieved modified Rankin Scale score 0-2. The treatment effect was not significant for small or large HVs. These findings have important trial design and therapeutic implications.
Registration: URL: https://www.
Clinicaltrials: gov; Unique identifier: NCT02175225.
Keywords: deferoxamine; hematoma; hemorrhage; iron; odds ratio.
Figures

References
-
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke. 1993;24:987–993 - PubMed
-
- Ruiz-Sandoval JL, Chiquete E, Romero-Vargas S, Padilla-Martinez JJ, Gonzalez-Cornejo S. Grading scale for prediction of outcome in primary intracerebral hemorrhages. Stroke. 2007;38:1641–1644 - PubMed
-
- Hegde A, Menon G, Kumar V. Surgery for spontaneous intracerebral hemorrhage - a comparative study with medical management in moderate to large sized hematomas. Clinical neurology and neurosurgery. 2019;184:105415. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

