VE-Cadherin Is Required for Cardiac Lymphatic Maintenance and Signaling
- PMID: 34789016
- PMCID: PMC8756423
- DOI: 10.1161/CIRCRESAHA.121.318852
VE-Cadherin Is Required for Cardiac Lymphatic Maintenance and Signaling
Abstract
Background: The adherens protein VE-cadherin (vascular endothelial cadherin) has diverse roles in organ-specific lymphatic vessels. However, its physiological role in cardiac lymphatics and its interaction with lymphangiogenic factors has not been fully explored. We sought to determine the spatiotemporal functions of VE-cadherin in cardiac lymphatics and mechanistically elucidate how VE-cadherin loss influences prolymphangiogenic signaling pathways, such as adrenomedullin and VEGF (vascular endothelial growth factor)-C/VEGFR3 (vascular endothelial growth factor receptor 3) signaling.
Methods: Cdh5flox/flox;Prox1CreERT2 mice were used to delete VE-cadherin in lymphatic endothelial cells across life stages, including embryonic, postnatal, and adult. Lymphatic architecture and function was characterized using immunostaining and functional lymphangiography. To evaluate the impact of temporal and functional regression of cardiac lymphatics in Cdh5flox/flox;Prox1CreERT2 mice, left anterior descending artery ligation was performed and cardiac function and repair after myocardial infarction was evaluated by echocardiography and histology. Cellular effects of VE-cadherin deletion on lymphatic signaling pathways were assessed by knockdown of VE-cadherin in cultured lymphatic endothelial cells.
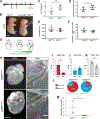
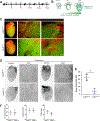
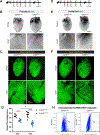
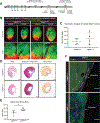
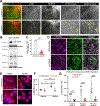
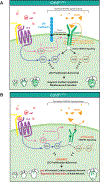
Results: Embryonic deletion of VE-cadherin produced edematous embryos with dilated cardiac lymphatics with significantly altered vessel tip morphology. Postnatal deletion of VE-cadherin caused complete disassembly of cardiac lymphatics. Adult deletion caused a temporal regression of the quiescent epicardial lymphatic network which correlated with significant dermal and cardiac lymphatic dysfunction, as measured by fluorescent and quantum dot lymphangiography, respectively. Surprisingly, despite regression of cardiac lymphatics, Cdh5flox/flox;Prox1CreERT2 mice exhibited preserved cardiac function, both at baseline and following myocardial infarction, compared with control mice. Mechanistically, loss of VE-cadherin leads to aberrant cellular internalization of VEGFR3, precluding the ability of VEGFR3 to be either canonically activated by VEGF-C or noncanonically transactivated by adrenomedullin signaling, impairing downstream processes such as cellular proliferation.
Conclusions: VE-cadherin is an essential scaffolding protein to maintain prolymphangiogenic signaling nodes at the plasma membrane, which are required for the development and adult maintenance of cardiac lymphatics, but not for cardiac function basally or after injury.
Keywords: adrenomedullin; cadherins; endothelial cells; myocardial infarction; vascular endothelial growth factor receptor.
Conflict of interest statement
DISCLOSURES
The authors declare that they have no conflicts of interest.
Figures

Comment in
-
VE-Cadherin: A Critical Sticking Point for Lymphatic System Maintenance: Role of VE-Cadherin in Lymphatic Maintenance.Circ Res. 2022 Jan 7;130(1):24-26. doi: 10.1161/CIRCRESAHA.121.320497. Epub 2022 Jan 7. Circ Res. 2022. PMID: 34995134 Free PMC article. No abstract available.
References
-
- Carmeliet P, Lampugnani MG, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert JM, Collen D, Dejana E. Targeted deficiency or cytosolic truncation of the ve-cadherin gene in mice impairs vegf-mediated endothelial survival and angiogenesis. Cell 1999;98:147–157 - PubMed
-
- Corada M, Mariotti M, Thurston G, Smith K, Kunkel R, Brockhaus M, Lampugnani MG, Martin-Padura I, Stoppacciaro A, Ruco L, McDonald DM, Ward PA, Dejana E. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci U S A 1999;96:9815–9820 - PMC - PubMed
-
- Lagendijk AK, Hogan BM. Ve-cadherin in vascular development: A coordinator of cell signaling and tissue morphogenesis. Curr Top Dev Biol 2015;112:325–352 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL142905/HL/NHLBI NIH HHS/United States
- F31 HL143836/HL/NHLBI NIH HHS/United States
- R01 DK119145/DK/NIDDK NIH HHS/United States
- P30 CA016086/CA/NCI NIH HHS/United States
- P30 NS045892/NS/NINDS NIH HHS/United States
- T32 GM133364/GM/NIGMS NIH HHS/United States
- R35 HL155656/HL/NHLBI NIH HHS/United States
- T32 HL069768/HL/NHLBI NIH HHS/United States
- R01 HL091973/HL/NHLBI NIH HHS/United States
- R01 HL129086/HL/NHLBI NIH HHS/United States
- T32 CA071341/CA/NCI NIH HHS/United States
- U54 HD079124/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

