Anticancer effects of mifepristone on human uveal melanoma cells
- PMID: 34789240
- PMCID: PMC8597220
- DOI: 10.1186/s12935-021-02306-y
Anticancer effects of mifepristone on human uveal melanoma cells
Abstract
Background: Uveal melanoma (UM), the most prevalent intraocular tumor in adults, is a highly metastatic and drug resistant lesion. Recent studies have demonstrated cytotoxic and anti-metastatic effects of the antiprogestin and antiglucocorticoid mifepristone (MF) in vitro and in clinical trials involving meningioma, colon, breast, and ovarian cancers. Drug repurposing is a cost-effective approach to bring approved drugs with good safety profiles to the clinic. This current study assessed the cytotoxic effects of MF in human UM cell lines of different genetic backgrounds.
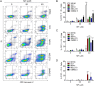
Methods: The effects of incremental concentrations of MF (0, 5, 10, 20, or 40 μM) on a panel of human UM primary (MEL270, 92.1, MP41, and MP46) and metastatic (OMM2.5) cells were evaluated. Cells were incubated with MF for up to 72 h before subsequent assays were conducted. Cellular functionality and viability were assessed by Cell Counting Kit-8, trypan blue exclusion assay, and quantitative label-free IncuCyte live-cell analysis. Cell death was analyzed by binding of Annexin V-FITC and/or PI, caspase-3/7 activity, and DNA fragmentation. Additionally, the release of cell-free DNA was assessed by droplet digital PCR, while the expression of progesterone and glucocorticoid receptors was determined by quantitative real-time reverse transcriptase PCR.
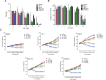
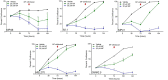
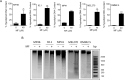
Results: MF treatment reduced cellular proliferation and viability of all UM cell lines studied in a concentration-dependent manner. A reduction in cell growth was observed at lower concentrations of MF, with evidence of cell death at higher concentrations. A significant increase in Annexin V-FITC and PI double positive cells, caspase-3/7 activity, DNA fragmentation, and cell-free DNA release suggests potent cytotoxicity of MF. None of the tested human UM cells expressed the classical progesterone receptor in the absence or presence of MF treatment, suggesting a mechanism independent of the modulation of the cognate nuclear progesterone receptor. In turn, all cells expressed non-classical progesterone receptors and the glucocorticoid receptor.
Conclusion: This study demonstrates that MF impedes the proliferation of UM cells in a concentration-dependent manner. We report that MF treatment at lower concentrations results in cell growth arrest, while increasing the concentration leads to lethality. MF, which has a good safety profile, could be a reliable adjuvant of a repurposing therapy against UM.
Keywords: Cancer therapy; Drug repurposing; Mifepristone; Uveal melanoma.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Chang AEKL, Menck HR. The National Cancer Data Base report on cutaneous and noncutaneous melanoma: a summary of 84,836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83(8):1664–1678. - PubMed
-
- Ghazawi FM, Darwich R, Le M, Rahme E, Zubarev A, Moreau L, Burnier JV, Sasseville D, Burnier MN, Litvinov IV. Uveal melanoma incidence trends in Canada: a national comprehensive population-based study. Br J Ophthalmol. 2019;103(12):1872–1876. - PubMed
-
- Rozeman EA, Dekker TJA, Haanen J, Blank CU. Advanced melanoma: current treatment options, biomarkers, and future perspectives. Am J Clin Dermatol. 2018;19(3):303–317. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

